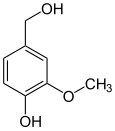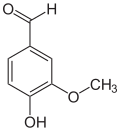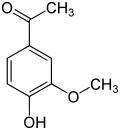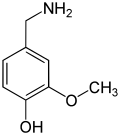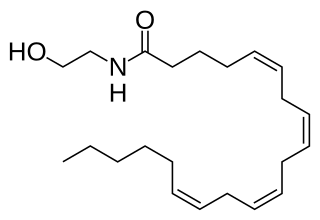
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), an N-acylethanolamine (NAE), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid receptors, the same receptors that the psychoactive compound THC in cannabis acts on. Anandamide is found in nearly all tissues in a wide range of animals. Anandamide has also been found in plants, including small amounts in chocolate. The name 'anandamide' is taken from the Sanskrit word ananda, which means "joy, bliss, delight", plus amide.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of tastes, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.

Resiniferatoxin (RTX) is a naturally occurring chemical found in resin spurge, a cactus-like plant commonly found in Morocco, and in Euphorbia poissonii found in northern Nigeria. It is a potent functional analog of capsaicin, the active ingredient in chili peppers.

The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the vertebrate central nervous system and peripheral nervous system. The endocannabinoid system remains under preliminary research, but may be involved in regulating physiological and cognitive processes, including fertility, pregnancy, pre- and postnatal development, various activity of immune system, appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis. The ECS plays an important role in multiple aspects of neural functions, including the control of movement and motor coordination, learning and memory, emotion and motivation, addictive-like behavior and pain modulation, among others.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.
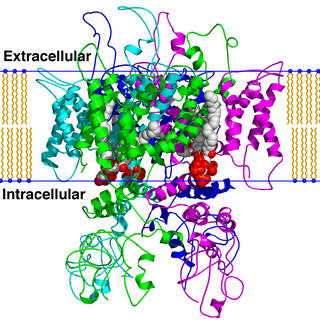
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.
AG 489 is a component of the venom produced by Agelenopsis aperta, a North American funnel web spider. It inhibits the ligand gated ion channel TRPV1 through a pore blocking mechanism.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.

G-protein coupled receptor 31 also known as 12-(S)-HETE receptor is a protein that in humans is encoded by the GPR31 gene. The human gene is located on chromosome 6q27 and encodes a G-protein coupled receptor protein composed of 319 amino acids.

N-Arachidonoyl dopamine (NADA) is an endocannabinoid that acts as an agonist of the CB1 receptor and the transient receptor potential V1 (TRPV1) ion channel. NADA was first described as a putative endocannabinoid (agonist for the CB1 receptor) in 2000 and was subsequently identified as an endovanilloid (agonist for TRPV1) in 2002. NADA is an endogenous arachidonic acid based lipid found in the brain of rats, with especially high concentrations in the hippocampus, cerebellum, and striatum. It activates the TRPV1 channel with an EC50 of approximately of 50 nM which makes it the putative endogenous TRPV1 agonist.

Iodoresiniferatoxin (I-RTX) is a strong competitive antagonist of the Transient Receptor Potential Vanilloid 1 (TRPV1) receptor. I-RTX is derived from resiniferatoxin (RTX).
Relief from chronic pain remains a recognized unmet medical need. Consequently, the search for new analgesic agents is being intensively studied by the pharmaceutical industry. The TRPV1 receptor is a ligand gated ion channel that has been implicated in mediation of many types of pain and therefore studied most extensively. The first competitive antagonist, capsazepine, was first described in 1990; since then, several TRPV1 antagonists have entered clinical trials as analgesic agents. Should these new chemical entities relieve symptoms of chronic pain, then this class of compounds may offer one of the first novel mechanisms for the treatment of pain in many years.
Capsinoids are alkaloid substances naturally present in chili peppers. Although they are structurally similar to capsaicin, the substance that causes pungency in hot peppers, they largely lack that characteristic. Capsinoids have an estimated "hot taste threshold" which is about 1/1000 that of capsaicin. Capsinoids were not reported in the scientific literature until 1989, when biologists first isolated them in a unique variety of chili peppers, CH-19 Sweet, which does not contain capsaicin. Capsinoids include capsiate, dihydrocapsiate, and nordihydrocapsiate.
Zucapsaicin (Civanex) is a medication used to treat osteoarthritis of the knee and other neuropathic pain. It is applied three times daily for a maximum of three months. Zucapsaicin is a member of phenols and a member of methoxybenzenes. It is a modulator of transient receptor potential cation channel subfamily V member 1 (TRPV-1), also known as the vanilloid or capsaicin receptor 1 that reduces pain, and improves articular functions. It is the cis-isomer of capsaicin. Civamide, manufactured by Winston Pharmaceuticals, is produced in formulations for oral, nasal, and topical use.

Arachidonoyl serotonin is an endogenous lipid signaling molecule. It was first described in 1998 as being an inhibitor of fatty acid amide hydrolase (FAAH). In 2007, it was shown to have analgesic properties and to act as an antagonist of the TRPV1 receptor. In 2011, it was shown to be present in the ileum and jejunum of the gastrointestinal tract and modulate glucagon-like peptide-1 (GLP-1) secretion. In addition to this, in 2016, AA-5-HT was also found to affect the signaling mechanisms responsible for anxiety, by inhibiting dopamine release from the Basolateral amygdala following fear behavior. In 2017, AA-5-HT was tested in its effects on the sleep wake cycle, where it was found to affect the sleep homeostasis when used in conjunction with molecules and chemicals that affect wake-related neurotransmitters.

LASSBio-881 is a drug which acts as both a non-selective partial agonist of the CB1 and CB2 cannabinoid receptors, and also as an antagonist of the TRPV1 receptor, as well as having antioxidant effects. It has potent anti-inflammatory and anti-hyperalgesic effects in animal studies.
RhTx is a small peptide toxin from Scolopendra subspinipes mutilans, also called the Chinese red-headed centipede. RhTx binds to the outer pore region of the temperature regulated TRPV1 ion channel, preferably in activated state, causing a downwards shift in the activation threshold temperature, which leads to the immediate onset of heat pain.

Phenylacetylrinvanil (IDN-5890) is a synthetic analogue of capsaicin which acts as a potent and selective agonist for the TRPV1 receptor, with slightly lower potency than resiniferatoxin, though still around 300 times the potency of capsaicin. It is an amide of vanillylamine and ricinoleic acid, with the hydroxyl group on ricinoleic acid esterified with phenylacetic acid. It is used to study the function of the TRPV1 receptor and its downstream actions, and has also shown anti-cancer effects in vitro.

Double-knot toxin (DkTx), also known as Tau-theraphotoxin-Hs1a or Tau-TRTX-Hs1a, is a toxin found in the venom of the Chinese Bird spider, a tarantula species primarily living in the Guangxi province of China. This toxin, characterized by its bivalent structure of two Inhibitor Cysteine Knots (ICK), is thought to induce excruciating and long-lasting pain by activating the transient receptor potential vanilloid 1 (TRPV1) channel.