Aconitum, also known as aconite, monkshood, wolf's-bane, leopard's bane, mousebane, women's bane, devil's helmet, queen of poisons, or blue rocket, is a genus of over 250 species of flowering plants belonging to the family Ranunculaceae. These herbaceous perennial plants are chiefly native to the mountainous parts of the Northern Hemisphere in North America, Europe, and Asia; growing in the moisture-retentive but well-draining soils of mountain meadows. Most species are extremely poisonous and must be dealt with very carefully. Several Aconitum hybrids, such as the Arendsii form of Aconitum carmichaelii, have won gardening awards — such as the Royal Horticultural Society’s Award of Garden Merit. Some are used by florists.

Campanula is one of several genera of flowering plants in the family Campanulaceae with the common name bellflower. It takes both its common and its scientific name from its bell-shaped flowers—campanula is Latin for "little bell".

Delphinidin is an anthocyanidin, a primary plant pigment, and also an antioxidant. Delphinidin gives blue hues to flowers in the genera Viola and Delphinium. It also gives the blue-red color of the grape that produces Cabernet Sauvignon, and can be found in cranberries and Concord grapes as well as pomegranates, and bilberries.

Ipomoea purpurea, the common morning-glory, tall morning-glory, or purple morning glory, is a species in the genus Ipomoea, native to Mexico and Central America.

Lamium album, commonly called white nettle or white dead-nettle, is a flowering plant in the family Lamiaceae. It is native throughout Europe and Asia, growing in a variety of habitats from open grassland to woodland, generally on moist, fertile soils.

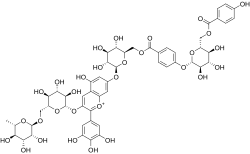
Malvin is a naturally occurring chemical of the anthocyanin family.

Aconitum carmichaelii syn. A. fischeri, is a species of flowering plant of the genus Aconitum, family Ranunculaceae. It is native to East Asia and eastern Russia. It is commonly known as Chinese aconite, Carmichael's monkshood or Chinese wolfsbane. In Mandarin Chinese, it is known as fùzǐ and as wūtóu and in Japanese as torikabuto.

Clitoria ternatea, commonly known as Asian pigeonwings, bluebellvine, blue pea, butterfly pea, cordofan pea and Darwin pea, is a plant species belonging to the family Fabaceae.

A metalloanthocyanin is a chemical complex giving color to petals of certain plants.

A xanthonoid is a chemical natural phenolic compound formed from the xanthone backbone. Many members of the Clusiaceae contain xanthonoids.
Psoralea plicata is a herb species in the genus Psoralea found in Pakistan.

Eusiderin is a neolignan found in Virola sp and Aniba sp.
Erythrina orientalis is a plant species in the genus Erythrina. This plant is a climbing herb that grows up to 6 m long, and has compound leaves with petioles that are 5–6 cm long. Its leaflets emerge in groups of three, and are 7–9 cm long and 5–8 cm wide. The plant's young leaves, flowers and pods are consumed as vegetables.

Lunularin is a dihydrostilbenoid found in common celery. It has also been found in the roots of Hydrangea macrophylla.

Hypolaetin is a flavone. It is the aglycone of hypolaetin 8-glucuronide, a compound found in the liverwort Marchantia berteroana. Hypolaetin 8-glucoside can be found in Sideritis leucantha.

Catechin 5-O-glucoside is a flavanol glucoside. It can be found in rhubarb and in the bark of Rhaphiolepis umbellata. It can also be formed from (+)-catechin by plant-cultured cells of Eucalyptus perriniana.

Isorhapontin is a stilbenoid. It is the glucoside of isorhapontigenin. It can be found in mycorrhizal and non-mycorrhizal roots of Norway spruces, in the bark of Picea sitchensis or in white spruce.
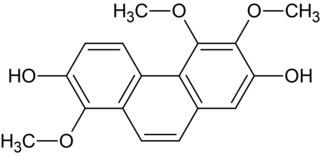
Confusarin is a phenanthrenoid found in the orchids Eria confusa and Bulbophyllum reptans. It can also be synthesized.

Blue flower colour was always associated with something unusual and desired. Blue roses especially were assumed to be a dream that cannot be realised. Blue colour in flower petals is caused by anthocyanins, which are members of flavonoid class metabolites. We can diversify three main classes of anthocyanin pigments: cyaniding type responsible for red coloration, pelargonidin type responsible for orange colour and delphinidin type responsible for violet/blue flower and fruits coloration. The main difference in the structure of listed anthocyanins type is the number of hydroxyl groups in the B-ring of the anthocyanin. Nevertheless in the monomeric state anthocyanins never show blue colour in the weak acidic and neutral pH. The mechanism of blue colour formation are very complicated in most cases, presence of delphinidin type pigments is not sufficient, great role play also the pH and the formation of complexes of anthocyanins with flavones and metal ions.
White flower colour is related to the absence or reduction of the anthocyanidin content. Unlike other colors, white colour is not induced by pigments. Several white plant tissues are principally equipped with the complete machinery for anthocyanin biosynthesis including the expression of regulatory genes. Nevertheless, they are unable to accumulate red or blue pigments, for example Dahlia ´Seattle´ petals showing a white tip. Several studies have revealed a further reduction of the anthocyanidin to colorless epicatechin by the enzyme anthocyanidin reductase (ANR).