Related Research Articles

Ash or ashes are the solid remnants of fires. Specifically, ash refers to all non-aqueous, non-gaseous residues that remain after something burns. In analytical chemistry, to analyse the mineral and metal content of chemical samples, ash is the non-gaseous, non-liquid residue after complete combustion.

Gallium is a chemical element; it has symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875, gallium is in group 13 of the periodic table and is similar to the other metals of the group.

Germanium is a chemical element; it has symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors silicon and tin. Like silicon, germanium naturally reacts and forms complexes with oxygen in nature.

The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process.

Chalcopyrite ( KAL-kə-PY-ryte, -koh-) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green-tinged black.

Anthracite, also known as hard coal and black coal, is a hard, compact variety of coal that has a submetallic lustre. It has the highest carbon content, the fewest impurities, and the highest energy density of all types of coal and is the highest ranking of coals.

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.

A briquette is a compressed block of coal dust or other combustible biomass material used for fuel and kindling to start a fire. The term derives from the French word brique, meaning brick.
In semiconductor production, doping is the intentional introduction of impurities into an intrinsic (undoped) semiconductor for the purpose of modulating its electrical, optical and structural properties. The doped material is referred to as an extrinsic semiconductor.

Silver bromide (AgBr) is a soft, pale-yellow, water-insoluble salt well known for its unusual sensitivity to light. This property has allowed silver halides to become the basis of modern photographic materials. AgBr is widely used in photographic films and is believed by some to have been used for making the Shroud of Turin. The salt can be found naturally as the mineral bromargyrite.

Germanium tetrachloride is a colourless, fuming liquid with a peculiar, acidic odour. It is used as an intermediate in the production of purified germanium metal. In recent years, GeCl4 usage has increased substantially due to its use as a reagent for fiber optic production.

Soil contamination, soil pollution, or land pollution as a part of land degradation is caused by the presence of xenobiotic (human-made) chemicals or other alteration in the natural soil environment. It is typically caused by industrial activity, agricultural chemicals or improper disposal of waste. The most common chemicals involved are petroleum hydrocarbons, polynuclear aromatic hydrocarbons, solvents, pesticides, lead, and other heavy metals. Contamination is correlated with the degree of industrialization and intensity of chemical substance. The concern over soil contamination stems primarily from health risks, from direct contact with the contaminated soil, vapour from the contaminants, or from secondary contamination of water supplies within and underlying the soil. Mapping of contaminated soil sites and the resulting clean ups are time-consuming and expensive tasks, and require expertise in geology, hydrology, chemistry, computer modelling, and GIS in Environmental Contamination, as well as an appreciation of the history of industrial chemistry.
Germanium dioxide, also called germanium(IV) oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula GeO2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen.
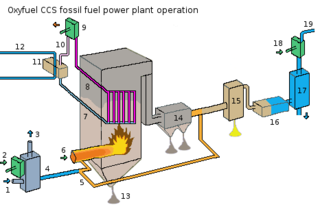
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology.

Coal combustion products (CCPs), also called coal combustion wastes (CCWs) or coal combustion residuals (CCRs), are categorized in four groups, each based on physical and chemical forms derived from coal combustion methods and emission controls:

A coal-fired power station or coal power plant is a thermal power station which burns coal to generate electricity. Worldwide there are over 2,400 coal-fired power stations, totaling over 2,000 gigawatts capacity. They generate about a third of the world's electricity, but cause many illnesses and the most early deaths, mainly from air pollution.

Leaching is the process of a solute becoming detached or extracted from its carrier substance by way of a solvent.

An ash pond, also called a coal ash basin or surface impoundment, is an engineered structure used at coal-fired power stations for the disposal of two types of coal combustion products: bottom ash and fly ash. The pond is used as a landfill to prevent the release of ash into the atmosphere. Although the use of ash ponds in combination with air pollution controls decreases the amount of airborne pollutants, the structures pose serious health risks for the surrounding environment.
Black Creek is a tributary of the Susquehanna River in Luzerne County, Pennsylvania, in the United States. It is approximately 2.6 miles (4.2 km) long and flows through Conyngham Township. The creek's watershed has an area of 3.85 square miles (10.0 km2). It is designated as a Coldwater Fishery and a Migratory Fishery. The creek is ephemeral and loses its flow to underground mines. Varying concentrations of many alkali metals, alkaline earth metals, and transition metals occur in water in the creek's watershed. The watershed typically experiences relatively mild temperatures. It is mainly accessible via U.S. Route 11, Pennsylvania Route 239, and a local road.
References
- ↑ "Vitrain | Railway, Transportation, Logistics | Britannica". www.britannica.com. Retrieved 2023-08-12.
- ↑ "Vitrain - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2023-08-12.
- ↑ "Vitrain: Mineral information, data and localities". MinDat. 19 June 2023. Retrieved 11 August 2023.
- ↑ Stadnichenko, Taisia (1953). "Concentration of Germanium in the Ash of American Coals: A Progress Report" (PDF). Geological Survey Circular (272) – via USGS.gov.