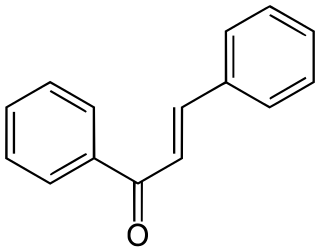
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids. They are widely known bioactive substances, fluorescent materials, and chemical intermediates.
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 that the "word synthon has now come to be used to mean synthetic building block rather than retrosynthetic fragmentation structures". It was noted in 1998 that the phrase did not feature very prominently in Corey's 1981 book The Logic of Chemical Synthesis, as it was not included in the index. Because synthons are charged, when placed into a synthesis an uncharged form is found commercially instead of forming and using the potentially very unstable charged synthons.

2-Imidazoline (Preferred IUPAC name: 4,5-dihydro-1H-imidazole) is one of three isomers of the nitrogen-containing heterocycle imidazoline, with the formula C3H6N2. The 2-imidazolines are the most common imidazolines commercially, as the ring exists in some natural products and some pharmaceuticals. They also have been examined in the context of organic synthesis, coordination chemistry, and homogeneous catalysis.

(+)-CPCA is a stimulant drug similar in structure to pethidine and to RTI-31, but nocaine is lacking the two-carbon bridge of RTI-31's tropane skeleton. This compound was first developed as a substitute agent for cocaine.
The Cook–Heilbron thiazole synthesis highlights the formation of 5-aminothiazoles through the chemical reaction of α-aminonitriles or aminocyanoacetates with dithioacids, carbon disulphide, carbon oxysulfide, or isothiocyanates at room temperature and under mild or aqueous conditions. Variation of substituents at the 2nd and 4th position of the thiazole is introduced by selecting different combinations of starting reagents.

Renin inhibitors are pharmaceutical drugs inhibiting the activity of renin that is responsible for hydrolyzing angiotensinogen to angiotensin I, which in turn reduces the formation of angiotensin II that facilitates blood pressure.

Himbacine is an alkaloid isolated from the bark of Australian magnolias. Himbacine has been synthesized using a Diels-Alder reaction as a key step. Himbacine's activity as a muscarinic receptor antagonist, with specificity for the muscarinic acetylcholine receptor M2, made it a promising starting point in Alzheimer's disease research. The development of a muscarinic antagonist based on himbacine failed but an analog, vorapaxar, has been approved by the FDA as a thrombin receptor antagonist.

2-Butyl-3-(p-tolyl)quinuclidine (BTQ) is a stimulant DRI. It is one of a number of substituted quinuclidine derivatives developed as potential medications for the treatment of cocaine abuse, and produces similar effects to cocaine in animal studies, although milder and longer-lasting.
Dipeptidyl peptidase-4 inhibitors are enzyme inhibitors that inhibit the enzyme dipeptidyl peptidase-4 (DPP-4). They are used in the treatment of type 2 diabetes mellitus. Inhibition of the DPP-4 enzyme prolongs and enhances the activity of incretins that play an important role in insulin secretion and blood glucose control regulation. Type 2 diabetes mellitus is a chronic metabolic disease that results from inability of the β-cells in the pancreas to secrete sufficient amounts of insulin to meet the body's needs. Insulin resistance and increased hepatic glucose production can also play a role by increasing the body's demand for insulin. Current treatments, other than insulin supplementation, are sometimes not sufficient to achieve control and may cause undesirable side effects, such as weight gain and hypoglycemia. In recent years, new drugs have been developed, based on continuing research into the mechanism of insulin production and regulation of the metabolism of sugar in the body. The enzyme DPP-4 has been found to play a significant role.
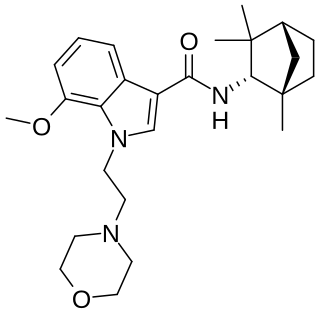
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.

Eudistomins are β-carboline derivatives, isolated from ascidians, like Ritterella sigillinoides, Lissoclinum fragile, or Pseudodistoma aureum.
Mark S. Cushman is an American chemist, whose primary research is in the area of medicinal chemistry. He completed his pre-pharmacy studies at Fresno State College (now California State University, Fresno) in 1965. He then attended the University of California San Francisco (as a University of California Regents Scholar), earning a Pharm.D. in 1969 and a Ph.D. in Medicinal Chemistry in 1973. Thereafter, he performed postdoctoral training in the laboratory of George Büchi, Ph.D., at the Massachusetts Institute of Technology (MIT). There, his research focused on the discovery and development of new synthetic methodologies, and the isolation and structural characterization of mycotoxins from Aspergillus niger. In 1975, he joined the Department of Medicinal Chemistry and Molecular Pharmacology (at the time, Department of Medicinal Chemistry and Pharmacognosy) at Purdue University. From 1983 to 1984, Prof. Cushman was a Senior Fulbright Scholar at Munich Technical University working in the laboratory of Professor Adelbert Bacher. His sabbatical work dealt with the design and synthesis of probes to elucidate key aspects of the biosynthesis of riboflavin (vitamin B2). Currently he holds the rank of Distinguished Professor Emeritus of Medicinal Chemistry at Purdue University. He has mentored 40 graduate students, 59 postdoctoral researchers, and 5 visiting scholars. He has published 348 papers and holds 41 patents. His work has ~17,000 citations with an h-index of 69. His most cited papers had 471, 403, and 299 citations as of August 2021. He has made seminal contributions to the fields of synthetic and medicinal chemistry including the development of new synthetic methodologies, the synthesis of natural products, and the preparation of antivirals, antibacterials, and anticancer agents, and mechanism probes to understand the function of over thirty macromolecular targets. One of his main scientific contributions is the development of the indenoisoquinolines, molecules that inhibit the action of toposiomerase I (Top1) and stabilize the G-quadruplex in the Myc promoter. Three indenoisoquinolines designed and synthesized by his research group at Purdue University [indotecan (LMP 400), indimitecan (LMP 776), and LMP 744] demonstrated potent anticancer activity in vivo and have completed phase I clinical trials at the National Institutes of Health.
Dawn N. Ward is an American synthetic organic chemist and associate professor in chemistry at Stevenson University.

Nirmatrelvir is an antiviral medication developed by Pfizer which acts as an orally active 3C-like protease inhibitor. It is part of a nirmatrelvir/ritonavir combination used to treat COVID-19 and sold under the brand name Paxlovid.
The Fiesselmann thiophene synthesis is a name reaction in organic chemistry that allows for the generation of 3-hydroxy-2-thiophenecarboxylic acid derivatives from α,β-acetylenic esters with thioglycolic acid and its derivatives under the presence of a base. The reaction was developed by Hans Fiesselmann in the 1950s.
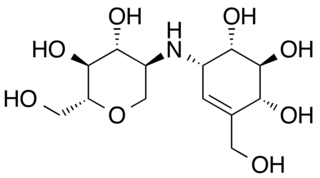
Salbostatin is an antibiotic and trehalase inhibitor with the molecular formula C13H23O8. Salbostatin is produced by the bacterium Streptomyces albus.
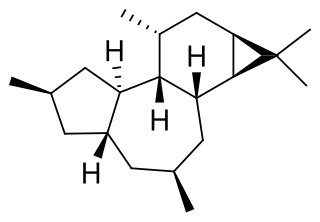
Tigliane is a diterpene that forms the structural basis for some natural chemical compounds such as phorbol.
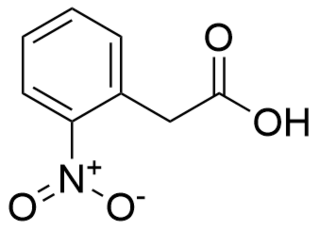
2-Nitrophenylacetic acid is an organic compound used in organic synthesis that has also been used as an herbicide. It is a derivative of phenylacetic acid, containing a phenyl functional group, a carboxylic acid functional group, and a nitro functional group. It is an important reagent for many organic reactions, especially for the formation of heterocycles.












