Related Research Articles

Flunitrazepam, also known as Rohypnol among other names, is a benzodiazepine used to treat severe insomnia and assist with anesthesia. As with other hypnotics, flunitrazepam has been advised to be prescribed only for short-term use or by those with chronic insomnia on an occasional basis. It is said to be 10 times more potent than diazepam.
Combinatorial chemistry comprises chemical synthetic methods that make it possible to prepare a large number of compounds in a single process. These compound libraries can be made as mixtures, sets of individual compounds or chemical structures generated by computer software. Combinatorial chemistry can be used for the synthesis of small molecules and for peptides.
Marquis reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of formaldehyde and concentrated sulfuric acid, which is dripped onto the substance being tested. The United States Department of Justice method for producing the reagent is the addition of 100 mL of concentrated (95–98%) sulfuric acid to 5 mL of 40% formaldehyde. Different compounds produce different color reactions. Methanol may be added to slow down the reaction process to allow better observation of the colour change.

Gas chromatography–mass spectrometry (GC-MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC-MS include drug detection, fire investigation, environmental analysis, explosives investigation, and identification of unknown samples, including that of material samples obtained from planet Mars during probe missions as early as the 1970s. GC-MS can also be used in airport security to detect substances in luggage or on human beings. Additionally, it can identify trace elements in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.
A drug test is a technical analysis of a biological specimen, for example urine, hair, blood, breath, sweat, and/or oral fluid/saliva—to determine the presence or absence of specified parent drugs or their metabolites. Major applications of drug testing include detection of the presence of performance enhancing steroids in sport, employers and parole/probation officers screening for drugs prohibited by law and police officers testing for the presence and concentration of alcohol (ethanol) in the blood commonly referred to as BAC. BAC tests are typically administered via a breathalyzer while urinalysis is used for the vast majority of drug testing in sports and the workplace. Numerous other methods with varying degrees of accuracy, sensitivity, and detection periods exist.
The Griess test is an analytical chemistry test which detects the presence of nitrite ion in solution. One of its most important uses is the determination of nitrite in drinking water. The Griess diazotization reaction, on which the Griess reagent relies, was first described in 1858 by Peter Griess. The test has also been widely used for the detection of nitrates, which are a common component of explosives, as they can be reduced to nitrites and detected with the Griess test.
Forensic chemistry is the application of chemistry and its subfield, forensic toxicology, in a legal setting. A forensic chemist can assist in the identification of unknown materials found at a crime scene. Specialists in this field have a wide array of methods and instruments to help identify unknown substances. These include high-performance liquid chromatography, gas chromatography-mass spectrometry, atomic absorption spectroscopy, Fourier transform infrared spectroscopy, and thin layer chromatography. The range of different methods is important due to the destructive nature of some instruments and the number of possible unknown substances that can be found at a scene. Forensic chemists prefer using nondestructive methods first, to preserve evidence and to determine which destructive methods will produce the best results.
Phenazepam is a benzodiazepine drug, which was developed in the Soviet Union in 1975, and now produced in Russia and some CIS countries.
Reagent testing is one of the processes used to identify substances contained within a pill, usually illicit substances. With the increased prevalence of drugs being available in their pure forms, the terms "drug checking" or "pill testing" may also be used, although these terms usually refer to testing with a wider variety of techniques covered by drug checking.

1-Fluoro-2,4-dinitrobenzene is a chemical that reacts with the N-terminal amino acid of polypeptides. This can be helpful for sequencing proteins.
para-Dimethylaminobenzaldehyde is an organic compound containing amine and aldehyde moieties which is used in Ehrlich's reagent and Kovac's reagent to test for indoles. The carbonyl group typically reacts with the electron rich 2-position of the indole but may also react at the C-3 or N-1 positions. It may also be used for determination of hydrazine.
Ehrlich's reagent or Ehrlich reagent is a reagent containing p-dimethylaminobenzaldehyde (DMAB) and thus can act as an indicator to presumptively identify indoles and urobilinogen. Several Ehrlich tests use the reagent in a medical test; some are drug tests and others contribute to diagnosis of various diseases or adverse drug reactions. It is named after Nobel Prize winner Paul Ehrlich who used it to distinguish typhoid from simple diarrhoea.
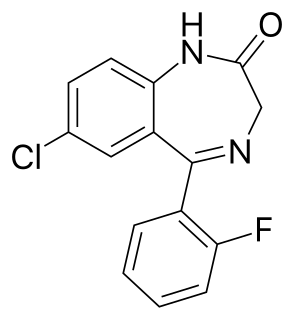
N-Desalkylflurazepam is a benzodiazepine analog and an active metabolite of several other benzodiazepine drugs including flurazepam, flutoprazepam, fludiazepam, midazolam, flutazolam, quazepam, and ethyl loflazepate. It is long-acting, prone to accumulation, and binds unselectively to the various benzodiazepine receptor subtypes. It has been sold as a designer drug from 2016 onward.
The Liebermann reagent named after Hungarian chemist Leo Liebermann (1852-1926) is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of potassium nitrite and concentrated sulfuric acid. 1 g of potassium nitrite is used for every 10 mL of sulfuric acid. Potassium nitrite may also be substituted by sodium nitrite. It is used to test for cocaine, morphine, PMA and PMMA.
The Mandelin reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of ammonium metavanadate and concentrated sulfuric acid. Its primary use is for the detection of ketamine and PMA Unlike the most common reagent test chemicals, it has a very strong yellow colour prior to being reacted with the unknown substance for testing. The colour of the reagent itself forms within about 48 hours of mixing and is important to factor into the colour-change-based testing method, as not all colour tables take care to start Mandelin results from this non-neutral yellow base hue.
Simon's reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It reacts with secondary amines like MDMA and methamphetamine to give a blue solution.
The Froehde reagent is used as a simple spot-test to presumptively identify alkaloids, especially opioids, as well as other compounds. It is composed of a mixture of molybdic acid or a molybdate salt dissolved in hot, concentrated sulfuric acid, which is then dripped onto the substance being tested.

Cannabis drug testing describes various drug test methodologies for the use of cannabis in medicine, sport, and law. Cannabis use is highly detectable and can be detected by urinalysis, hair analysis, as well as saliva tests for days or weeks.
The Gallic acid reagent is used as a simple spot-test to presumptively identify drug precursor chemicals. It is composed of a mixture of gallic acid and concentrated sulfuric acid.

Cloniprazepam is a benzodiazepine derivative and a prodrug mainly for clonazepam and other metabolites., including 7-aminoclonazepam and clonazepam mentioned above, which may be misinterpreted as clonazepam intake at the result of a drug test.
References
- ↑ Janák, editors, Zdeněk Deyl, Karel Macek, Jaroslav (1975). Liquid Column Chromatography a Survey of Modern Techniques and Applications. Burlington: Elsevier. p. 603. ISBN 9780080858036 . Retrieved 10 January 2016.CS1 maint: extra text: authors list (link)
- ↑ Steroid Dynamics: Proceedings of the Symposium on the Dynamics of Steroid Hormones Held in Tokyo, May, 1965. Academic Press. 31 January 2017. p. 381. ISBN 9781483270852 . Retrieved 10 January 2016.
- ↑ Callow, R.K.; Campbell, P.N.; Datta, S.P (2013). The Chromatography of Steroids: International Series of Monographs on Pure and Applied Biology: Biochemistry. Elsevier. pp. 232, 270. ISBN 9781483184579 . Retrieved 10 January 2016.
- ↑ "Recommended methods for the I dentification and A nalysis of Barbiturates and Benzodiazepines under International Control" (PDF). UNODC. 2012. p. 25. Retrieved 10 January 2016.
- ↑ "Rapid Testing Methods of Drugs of Abuse" (PDF). UNODC. 1994. p. 103. Retrieved 10 January 2016.
- ↑ 0-471-49252-3
- ↑ Kovar, Karl-Artur & Laudszun, Martina (February 1989). "Chemistry and Reaction Mechanisms of Rapid Tests for Drugs of Abuse and Precursors Chemicals" (PDF). UNODC. p. 13. Retrieved 3 January 2016.