
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, opacity, and luster, but may have properties that differ from those of the pure metals, such as increased strength or hardness. In some cases, an alloy may reduce the overall cost of the material while preserving important properties. In other cases, the mixture imparts synergistic properties to the constituent metal elements such as corrosion resistance or mechanical strength.

Brass is an alloy of copper and zinc, in proportions which can be varied to achieve different colours and mechanical, electrical, acoustic and chemical properties, but copper typically has the larger proportion. In use since prehistoric times, it is a substitutional alloy: atoms of the two constituents may replace each other within the same crystal structure.

Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.

Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), and is typically associated with the corrosion of refined iron.

Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.

Brazing is a metal-joining process in which two or more metal items are joined by melting and flowing a filler metal into the joint, with the filler metal having a lower melting point than the adjoining metal.

Die casting is a metal casting process that is characterized by forcing molten metal under high pressure into a mold cavity. The mold cavity is created using two hardened tool steel dies which have been machined into shape and work similarly to an injection mold during the process. Most die castings are made from non-ferrous metals, specifically zinc, copper, aluminium, magnesium, lead, pewter, and tin-based alloys. Depending on the type of metal being cast, a hot- or cold-chamber machine is used.

Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.

A die-cast toy is a toy or a collectible model produced by using the die-casting method of putting molten lead, zinc alloy or plastic in a mold to produce a particular shape. Such toys are made of metal, with plastic, rubber, glass, or other machined metal parts. Wholly plastic toys are made by a similar process of injection molding, but the two methods are distinct because of the properties of the materials.

ZAMAK is a family of alloys with a base metal of zinc and alloying elements of aluminium, magnesium, and copper.

Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material can strongly influence physical properties such as strength, toughness, ductility, hardness, corrosion resistance, high/low temperature behaviour or wear resistance. These properties in turn govern the application of these materials in industrial practice.

Pot metal is an alloy of low-melting point metals that manufacturers use to make fast, inexpensive castings. The term "pot metal" came about because of automobile factories' practice in the early 20th century of gathering up non-ferrous metal scraps from the manufacturing processes and melting them in one pot to form into cast products. Small amounts of iron often made it into the castings but never in significant quantity because too much iron would raise the melting point too high for simple casting operations.
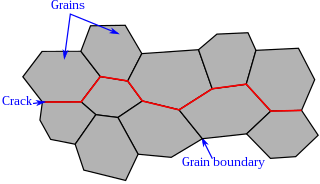
Intergranular fracture, intergranular cracking or intergranular embrittlement occurs when a crack propagates along the grain boundaries of a material, usually when these grain boundaries are weakened. The more commonly seen transgranular fracture, occurs when the crack grows through the material grains. As an analogy, in a wall of bricks, intergranular fracture would correspond to a fracture that takes place in the mortar that keeps the bricks together.
In materials science, environmental stress fracture or environment assisted fracture is the generic name given to premature failure under the influence of tensile stresses and harmful environments of materials such as metals and alloys, composites, plastics and ceramics.
Liquid metal embrittlement is a phenomenon of practical importance, where certain ductile metals experience drastic loss in tensile ductility or undergo brittle fracture when exposed to specific liquid metals. Generally, tensile stress, either externally applied or internally present, is needed to induce embrittlement. Exceptions to this rule have been observed, as in the case of aluminium in the presence of liquid gallium. This phenomenon has been studied since the beginning of the 20th century. Many of its phenomenological characteristics are known and several mechanisms have been proposed to explain it. The practical significance of liquid metal embrittlement is revealed by the observation that several steels experience ductility losses and cracking during hot-dip galvanizing or during subsequent fabrication. Cracking can occur catastrophically and very high crack growth rates have been measured.
Corrosion fatigue is fatigue in a corrosive environment. It is the mechanical degradation of a material under the joint action of corrosion and cyclic loading. Nearly all engineering structures experience some form of alternating stress, and are exposed to harmful environments during their service life. The environment plays a significant role in the fatigue of high-strength structural materials like steel, aluminum alloys and titanium alloys. Materials with high specific strength are being developed to meet the requirements of advancing technology. However, their usefulness depends to a large extent on the degree to which they resist corrosion fatigue.
Magnesium alloys are mixtures of magnesium with other metals, often aluminium, zinc, manganese, silicon, copper, rare earths and zirconium. Magnesium alloys have a hexagonal lattice structure, which affects the fundamental properties of these alloys. Plastic deformation of the hexagonal lattice is more complicated than in cubic latticed metals like aluminium, copper and steel; therefore, magnesium alloys are typically used as cast alloys, but research of wrought alloys has been more extensive since 2003. Cast magnesium alloys are used for many components of modern automobiles and have been used in some high-performance vehicles; die-cast magnesium is also used for camera bodies and components in lenses.
Zinc refining is the process of purifying zinc to special high grade (SHG) zinc, which is at least 99.995% pure. This process is not usually required when smelting of zinc is done through electrolysis processes, but is needed when zinc is produced by pyrometallurgical processes, because it is only 98.5% pure.
A casting defect is an undesired irregularity in a metal casting process. Some defects can be tolerated while others can be repaired, otherwise they must be eliminated. They are broken down into five main categories: gas porosity, shrinkage defects, mould material defects, pouring metal defects, and metallurgical defects.
Metallurgical failure analysis is the process to determine the mechanism that has caused a metal component to fail. It can identify the cause of failure, providing insight into the root cause and potential solutions to prevent similar failures in the future, as well as culpability, which is important in legal cases. Resolving the source of metallurgical failures can be of financial interest to companies. The annual cost of corrosion in the United States was estimated by NACE International in 2012 to be $450 billion a year, a 67% increase compared to estimates for 2001. These failures can be analyzed to determine their root cause, which if corrected, would save reduce the cost of failures to companies.
















