Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, and profit. Fungicides are used both in agriculture and to fight fungal infections in animals. Fungicides are also used to control oomycetes, which are not taxonomically/genetically fungi, although sharing similar methods of infecting plants. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upward. Most fungicides that can be bought retail are sold in liquid form, the active ingredient being present at 0.08% in weaker concentrates, and as high as 0.5% for more potent fungicides. Fungicides in powdered form are usually around 90% sulfur.

Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2·nH2O, with n ranging from 0 to 4.5, forming hydrates. Zinc chloride, anhydrous and its hydrates are colorless or white crystalline solids, and are highly soluble in water. Five hydrates of zinc chloride are known, as well as four forms of anhydrous zinc chloride. This salt is hygroscopic and even deliquescent. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
IARC group 3 substances, chemical mixtures and exposure circumstances are those that can not be classified in regard to their carcinogenicity to humans by the International Agency for Research on Cancer (IARC). This category is used most commonly for agents, mixtures and exposure circumstances for which the level of evidence of carcinogenicity is inadequate in humans and inadequate or limited in experimental animals. Exceptionally, agents (mixtures) for which the evidence of carcinogenicity is inadequate in humans, but sufficient in experimental animals may be placed in this category when there is strong evidence that the mechanism of carcinogenicity in experimental animals does not operate in humans. Agents, mixtures and exposure circumstances that do not fall into any other group are also placed in this category.

Sodium dithionite is a white crystalline powder with a sulfurous odor. Although it is stable in dry air, it decomposes in hot water and in acid solutions.

Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.

Rongalite is a chemical compound with the molecular formula Na+HOCH2SO2−. This salt has many additional names, including Rongalit, sodium hydroxymethylsulfinate, sodium formaldehyde sulfoxylate, and Bruggolite. It is listed in the European Cosmetics Directive as sodium oxymethylene sulfoxylate (INCI). It is water-soluble and generally sold as the dihydrate. The compound and its derivatives are widely used in the dye industry. The structure of this salt has been confirmed by X-ray crystallography.

Sodium diethyldithiocarbamate is the organosulfur compound with the formula NaS2CN(C2H5)2. It is a pale yellow, water soluble salt.

In organic chemistry, a dithiocarbamate is a functional group with the general formula R2N−C(=S)−S−R and structure >N−C(=S)−S−. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms.

Metam sodium is an organosulfur compound with the formula CH3NHCS2Na. The compound is a sodium salt of a dithiocarbamate. The compound exists as a colorless dihydrate, but most commonly it is encountered as an aqueous solution. It is used as a soil fumigant, pesticide, herbicide, and fungicide. It is one of the most widely used pesticides in the United States, with approximately 60 million pounds used in 2001.
In chemistry the term zincate may refer to several substances containing the element zinc:
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript appearance and behavior: they are generally colorless, do not readily engage in redox reactions, and generally adopt symmetrical structures.
This is an index of articles relating to pesticides.
| GHS labelling: |-

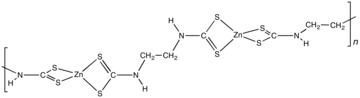
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications.

Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide.

2-Mercaptobenzothiazole is an organosulfur compound with the formula C6H4(NH)SC=S. A white solid, it is used in the sulfur vulcanization of rubber.
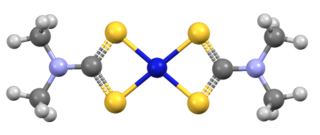
Nickel bis(dimethyldithiocarbamate) is the coordination complex on nickel and dimethyldithiocarbamate, with the formula Ni(S2CNMe2)2 (Me = methyl). It is the prototype for a large number of bis(dialkhyldithiocarbamate)s of nickel(II), which feature diverse organic substituents, all of which have similar structures. Nickel bis(dimethyldithiocarbamate) has been marketed as a fungicide and related complexes are used as stabilizers in polymers.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.