
Gilead Sciences, Inc. is an American biopharmaceutical company headquartered in Foster City, California that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and COVID-19, including ledipasvir/sofosbuvir and sofosbuvir. Gilead is a member of the NASDAQ Biotechnology Index and the S&P 500.
The pharmaceutical industry in India was valued at an estimated US$42 billion in 2021 and is estimated to reach $130 billion by 2030. India is the world's largest provider of generic medicines by volume, with a 20% share of total global pharmaceutical exports. It is also the largest vaccine supplier in the world by volume, accounting for more than 60% of all vaccines manufactured in the world. Indian pharmaceutical products are exported to various regulated markets including the US, UK, European Union and Canada.

Hetero Drugs is an Indian pharmaceutical company and the world's largest producer of anti-retroviral drugs. Hetero's business includes APIs, generics, biosimilars, custom pharmaceutical services, and branded generics.
Ramanbhai B. Patel was an Indian chemist who founded the operation, along with school friend I A Modi, that eventually became the Ahmedabad-based pharmaceutical company Cadila Laboratories.

Century Pharmaceuticals Ltd. is an Indian pharmaceutical company, founded in 1982 in Vadodara, Gujarat, India.
Serum Institute of India (SII) is an Indian biotechnology and biopharmaceuticals company, based in Pune. It was founded by Cyrus Poonawalla in 1966 and is a part of Cyrus Poonawalla Group.
Pankaj Ramanbhai Patel is an Indian billionaire businessman, and the chairman of Zydus Lifesciences, the fifth largest pharmaceutical company in India.

Intas Pharmaceuticals Limited is an Indian multinational pharmaceutical company headquartered in Ahmedabad. It is a producer of generic therapeutic drugs and engaged in contract clinical research and manufacturing. It has 22 manufacturing plants, 17 in India and the rest in Greece, United Kingdom and Mexico. In the financial year 2019, 69% of the company's revenue came from international markets while 31% came from India. It's market presence is more than 100+ countries.

Simeprevir, sold under the brand name Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C. It is specifically used for hepatitis C genotype 1 and 4. Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa. Cure rates are in 80s to 90s percent. It may be used in those who also have HIV/AIDS. It is taken by mouth once daily for typically 12 weeks.

Saroglitazar is a drug for the treatment of type 2 diabetes mellitus, dyslipidemia, NASH and NAFLD It is approved for use in India by the Drug Controller General of India. Saroglitazar is indicated for the treatment of diabetic dyslipidemia and hypertriglyceridemia with type 2 diabetes mellitus not controlled by statin therapy. In clinical studies, saroglitazar has demonstrated reduction of triglycerides (TG), LDL cholesterol, VLDL cholesterol, non-HDL cholesterol and an increase in HDL cholesterol a characteristic hallmark of atherogenic diabetic dyslipidemia (ADD). It has also shown anti-diabetic medication properties by reducing the fasting plasma glucose and HBA1c in diabetes patients.

Cadila Pharmaceuticals is an Indian multinational pharmaceutical company based in Ahmedabad. The company's operations focus on manufacturing products ranging from active pharmaceutical intermediates, finished formulations, food supplements, biotechnology products and pharmaceutical machinery.
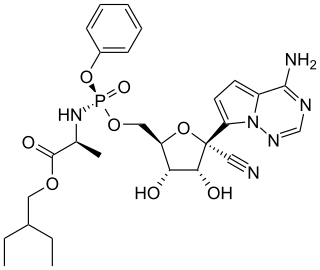
Remdesivir, sold under the brand name Veklury, is a broad-spectrum antiviral medication developed by the biopharmaceutical company Gilead Sciences. It is administered via injection into a vein. During the COVID‑19 pandemic, remdesivir was approved or authorized for emergency use to treat COVID‑19 in numerous countries.
Sofosbuvir/velpatasvir, sold under the brand name Epclusa among others, is a fixed-dose combination medication for the treatment of hepatitis C in adults. It combines sofosbuvir and velpatasvir. It is more than 90% effective for hepatitis C genotypes one through six. It also works for hepatitis C in those who also have cirrhosis or HIV/AIDS. It is taken by mouth.
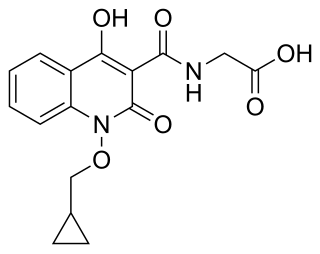
Desidustat is a drug for the treatment of anemia of chronic kidney disease. This drug with the brand name Oxemia is discovered and developed by Zydus Life Sciences. Desidustat reduces the requirement of recombinant erythropoietin requirement in anemia, and decreases EPO-resistance, by reducing IL-6, IL-1β, and anti-EPO antibodies. The subject expert committee of CDSCO has recommended the grant of permission for manufacturing and marketing of Desidustat 25 mg and 50 mg tablets in India,based on some conditions related to package insert, phase 4 protocols, prescription details, and GCP. Clinical trials on desidustat have been done in India and Australia. In a Phase 2, randomized, double-blind, 6-week, placebo-controlled, dose-ranging, safety and efficacy study, a mean hemoglobin increase of 1.57, 2.22, and 2.92 g/dL in desidustat 100, 150, and 200 mg arms, respectively, was observed. The Phase 3 clinical trials were conducted in chronic kidney disease patients which were not on dialysis as well as on dialysis. Desidustat is developed for the treatment of anemia as an oral tablet, where currently injections of erythropoietin and its analogues are drugs of choice. Desidustat is a HIF prolyl-hydroxylase inhibitor. In preclinical studies, effects of desidustat was assessed in normal and nephrectomized rats, and in chemotherapy-induced anemia. Desidustat demonstrated hematinic potential by combined effects on endogenous erythropoietin release and efficient iron utilization. Desidustat can also be useful in treatment of anemia of inflammation since it causes efficient erythropoiesis and hepcidin downregulation. In January 2020, Zydus entered into licensing agreement with China Medical System (CMS) Holdings for development and commercialization of desidustat in Greater China. Under the license agreement, CMS will pay Zydus an initial upfront payment, regulatory milestones, sales milestones and royalties on net sales of the product. CMS will be responsible for development, registration and commercialization of desidustat in Greater China. National Medical Products Administration of China (NMPA) accepted the new drug application for desidustat on 23rd April 2024. It has been observed that desidustat protects against acute and chronic kidney injury by reducing inflammatory cytokines like IL-6 and oxidative stress. A clinical trial to evaluate the efficacy and safety of desidustat tablet for the management of COVID-19 patients is ongoing in Mexico, wherein desidustat has shown to prevent acute respiratory distress syndrome (ARDS) by inhibiting IL-6. Zydus has also received approval from the US FDA to initiate clinical trials of desidustat in chemotherapy Induced anemia (CIA). Zydus Lifesciences and Sun Pharmaceuticals have entered an agreement in October 2023 to co-market Desidustat. Sun Pharma will sell the drug as Rytstat, while Zydus will continue to sell it as Oxemia.

The Solidarity trial for treatments is a multinational Phase III-IV clinical trial organized by the World Health Organization (WHO) and partners to compare four untested treatments for hospitalized people with severe COVID-19 illness. The trial was announced 18 March 2020, and as of 6 August 2021, 12,000 patients in 30 countries had been recruited to participate in the trial.

Zydus Wellness is an Indian consumer goods company headquartered in Ahmedabad, which produces nutrition and skincare products. It is a subsidiary of the pharmaceutical company Zydus Lifesciences. Its brands include Glucon-D, Sugar Free, EverYuth, Complan, and Nycil. The company operates three manufacturing plants, one in Gujarat and 2 in Sikkim.

Bharat Biotech International Limited (BBIL) is an Indian multinational biotechnology company based in Hyderabad, which is engaged in drug discovery, drug development, and the manufacture of vaccines, biotherapeutics, pharmaceuticals and healthcare products.

India began administration of COVID-19 vaccines on 16 January 2021. As of 4 March 2023, India has administered over 2.2 billion doses overall, including first, second and precautionary (booster) doses of the currently approved vaccines. In India, 95% of the eligible population (12+) has received at least one shot, and 88% of the eligible population (12+) is fully vaccinated.

ZyCoV-D is a DNA plasmid-based COVID-19 vaccine developed by Indian pharmaceutical company Cadila Healthcare, with support from the Biotechnology Industry Research Assistance Council. It is approved for emergency use in India.
The Pharmaceutical industry in Gujarat ranks number one in India with a 33% share in drug manufacturing and a 28% share in drug exports. The state has 130 USFDA certified drug manufacturing facilities. Ahmedabad and Vadodara are considered as pharmaceutical hubs as there are many big and small pharma companies established in these cities.














