
In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.
Chromic acid is jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.

An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.

A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure R−CH2−C6H5. Benzyl features a benzene ring attached to a methylene group group.

Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.
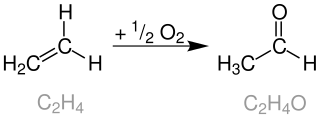
The Wacker process or the Hoechst-Wacker process refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride and copper(II) chloride as the catalyst. This chemical reaction was one of the first homogeneous catalysis with organopalladium chemistry applied on an industrial scale.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.
Calcium hypochlorite is an inorganic compound with chemical formula Ca(ClO)2, also written as Ca(OCl)2. It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical. It is the main active ingredient of commercial products called bleaching powder, used for water treatment and as a bleaching agent.
The Corey–Kim oxidation is an oxidation reaction used to synthesise aldehydes and ketones from primary and secondary alcohols. It is named for American chemist and Nobel Laureate Elias James Corey and Korean-American chemist Choung Un Kim.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.
Ruthenium tetroxide is the inorganic compound with the formula RuO4. It is a yellow volatile solid that melts near room temperature. It has the odor of ozone. Samples are typically black due to impurities. The analogous OsO4 is more widely used and better known. It is also the anhydride of hyperruthenic acid (H2RuO5). One of the few solvents in which RuO4 forms stable solutions is CCl4.

The Sarett oxidation is an organic reaction that oxidizes primary and secondary alcohols to aldehydes and ketones, respectively, using chromium trioxide and pyridine. Unlike the similar Jones oxidation, the Sarett oxidation will not further oxidize primary alcohols to their carboxylic acid form, neither will it affect carbon-carbon double bonds. Use of the original Sarett oxidation has become largely antiquated however, in favor of other modified oxidation techniques. The unadulterated reaction is still occasionally used in teaching settings and in small scale laboratory research.

The Ojima lactam is an organic compound of some importance in the commercial production of Taxol. This lactam was first synthesized by Iwao Ojima. The organic synthesis is an illustration of asymmetric synthesis via a chiral auxiliary.
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.
Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters where the carbon carries a higher oxidation state. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.
The Pinnick oxidation is an organic reaction by which aldehydes can be oxidized into their corresponding carboxylic acids using sodium chlorite (NaClO2) under mild acidic conditions. It was originally developed by Lindgren and Nilsson. The typical reaction conditions used today were developed by G. A. Kraus. H.W. Pinnick later demonstrated that these conditions could be applied to oxidize α,β-unsaturated aldehydes. There exist many different reactions to oxidize aldehydes, but only a few are amenable to a broad range of functional groups. The Pinnick oxidation has proven to be both tolerant of sensitive functionalities and capable of reacting with sterically hindered groups. This reaction is especially useful for oxidizing α,β-unsaturated aldehydes, and another one of its advantages is its relatively low cost.
Jiro Tsuji was a Japanese chemist, notable for his discovery of organometallic reactions, including the Tsuji-Trost reaction, the Tsuji-Wilkinson decarbonylation, and the Tsuji-Wacker reaction.











