Bioterrorism is terrorism involving the intentional release or dissemination of biological agents. These agents include bacteria, viruses, insects, fungi, and/or their toxins, and may be in a naturally occurring or a human-modified form, in much the same way as in biological warfare. Further, modern agribusiness is vulnerable to anti-agricultural attacks by terrorists, and such attacks can seriously damage economy as well as consumer confidence. The latter destructive activity is called agrobioterrorism and is a subtype of agro-terrorism.

Vaccination is the administration of a vaccine to help the immune system develop immunity from a disease. Vaccines contain a microorganism or virus in a weakened, live or killed state, or proteins or toxins from the organism. In stimulating the body's adaptive immunity, they help prevent sickness from an infectious disease. When a sufficiently large percentage of a population has been vaccinated, herd immunity results. Herd immunity protects those who may be immunocompromised and cannot get a vaccine because even a weakened version would harm them. The effectiveness of vaccination has been widely studied and verified. Vaccination is the most effective method of preventing infectious diseases; widespread immunity due to vaccination is largely responsible for the worldwide eradication of smallpox and the elimination of diseases such as polio and tetanus from much of the world. However, some diseases, such as measles outbreaks in America, have seen rising cases due to relatively low vaccination rates in the 2010s – attributed, in part, to vaccine hesitancy. According to the World Health Organization, vaccination prevents 3.5–5 million deaths per year.

A vaccine is a biological preparation that provides active acquired immunity to a particular infectious or malignant disease. The safety and effectiveness of vaccines has been widely studied and verified. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. The agent stimulates the body's immune system to recognize the agent as a threat, destroy it, and recognize further and destroy any of the microorganisms associated with that agent that it may encounter in the future.

Measles is a highly contagious, vaccine-preventable infectious disease caused by measles virus. Symptoms usually develop 10–12 days after exposure to an infected person and last 7–10 days. Initial symptoms typically include fever, often greater than 40 °C (104 °F), cough, runny nose, and inflamed eyes. Small white spots known as Koplik's spots may form inside the mouth two or three days after the start of symptoms. A red, flat rash which usually starts on the face and then spreads to the rest of the body typically begins three to five days after the start of symptoms. Common complications include diarrhea, middle ear infection (7%), and pneumonia (6%). These occur in part due to measles-induced immunosuppression. Less commonly seizures, blindness, or inflammation of the brain may occur. Other names include morbilli, rubeola, red measles, and English measles. Both rubella, also known as German measles, and roseola are different diseases caused by unrelated viruses.

The smallpox vaccine is the first vaccine to have been developed against a contagious disease. In 1796, British physician Edward Jenner demonstrated that an infection with the relatively mild cowpox virus conferred immunity against the deadly smallpox virus. Cowpox served as a natural vaccine until the modern smallpox vaccine emerged in the 20th century. From 1958 to 1977, the World Health Organization (WHO) conducted a global vaccination campaign that eradicated smallpox, making it the only human disease to be eradicated. Although routine smallpox vaccination is no longer performed on the general public, the vaccine is still being produced to guard against bioterrorism, biological warfare, and mpox.

Mpox is an infectious viral disease that can occur in humans and other animals. Symptoms include a rash that forms blisters and then crusts over, fever, and swollen lymph nodes. The illness is usually mild and most of those infected will recover within a few weeks without treatment. The time from exposure to onset of symptoms ranges from five to twenty-one days and symptoms typically last from two to four weeks. Cases may be severe, especially in children, pregnant women or people with suppressed immune systems.

Vaccinia virus is a large, complex, enveloped virus belonging to the poxvirus family. It has a linear, double-stranded DNA genome approximately 190 kbp in length, which encodes approximately 250 genes. The dimensions of the virion are roughly 360 × 270 × 250 nm, with a mass of approximately 5–10 fg. The vaccinia virus is the source of the modern smallpox vaccine, which the World Health Organization (WHO) used to eradicate smallpox in a global vaccination campaign in 1958–1977. Although smallpox no longer exists in the wild, vaccinia virus is still studied widely by scientists as a tool for gene therapy and genetic engineering.

Donald Ainslie Henderson was an American medical doctor, educator, and epidemiologist who directed a 10-year international effort (1967–1977) that eradicated smallpox throughout the world and launched international childhood vaccination programs. From 1977 to 1990, he was Dean of the Johns Hopkins School of Public Health. Later, he played a leading role in instigating national programs for public health preparedness and response following biological attacks and national disasters. At the time of his death, he was Professor and Dean Emeritus of the Johns Hopkins Bloomberg School of Public Health, and Professor of Medicine and Public Health at the University of Pittsburgh, as well as Distinguished Scholar at the UPMC Center for Health Security.
The MMRV vaccine combines the attenuated virus MMR vaccine with the addition of the varicella (chickenpox) vaccine. The MMRV vaccine is typically given to children between one and two years of age.
Varicella vaccine, also known as chickenpox vaccine, is a vaccine that protects against chickenpox. One dose of vaccine prevents 95% of moderate disease and 100% of severe disease. Two doses of vaccine are more effective than one. If given to those who are not immune within five days of exposure to chickenpox it prevents most cases of disease. Vaccinating a large portion of the population also protects those who are not vaccinated. It is given by injection just under the skin. Another vaccine, known as zoster vaccine, is used to prevent diseases caused by the same virus – the varicella zoster virus.
Mass vaccination is a public policy effort to vaccinate a large number of people, possibly the entire population of the world or of a country or region, within a short period of time. This policy may be directed during a pandemic, when there is a localized outbreak or scare of a disease for which a vaccine exists, or when a new vaccine is invented.
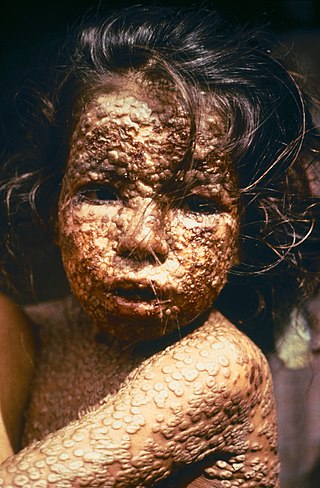
Smallpox was an infectious disease caused by variola virus, which belongs to the genus Orthopoxvirus. The last naturally occurring case was diagnosed in October 1977, and the World Health Organization (WHO) certified the global eradication of the disease in 1980, making smallpox the only human disease to have been eradicated to date.

Yellow fever vaccine is a vaccine that protects against yellow fever. Yellow fever is a viral infection that occurs in Africa and South America. Most people begin to develop immunity within ten days of vaccination and 99% are protected within one month, and this appears to be lifelong. The vaccine can be used to control outbreaks of disease. It is given either by injection into a muscle or just under the skin.

The 2009 swine flu pandemic vaccines were influenza vaccines developed to protect against the pandemic H1N1/09 virus. These vaccines either contained inactivated (killed) influenza virus, or weakened live virus that could not cause influenza. The killed virus was injected, while the live virus was given as a nasal spray. Both these types of vaccine were produced by growing the virus in chicken eggs. Around three billion doses were produced, with delivery in November 2009.
Operation Dark Winter was the code name for a senior-level bio-terrorist attack simulation conducted on June 22–23, 2001. It was designed to carry out a mock version of a covert and widespread smallpox attack on the United States. Tara O'Toole and Tom Inglesby of the Johns Hopkins Center for Civilian Biodefense Strategies (CCBS) / Center for Strategic and International Studies (CSIS), and Randy Larsen and Mark DeMier of Analytic Services were the principal designers, authors, and controllers of the Dark Winter project.
Vaccinia immune globulin (VIG) is made from the pooled blood of individuals who have been inoculated with the smallpox vaccine. The antibodies these individuals developed in response to the smallpox vaccine are removed and purified. This results in VIG. It can be administered intravenously. It is used to treat individuals who have developed progressive vaccinia after smallpox vaccination.

Ring vaccination is a strategy to inhibit the spread of a disease by vaccinating those who are most likely to be infected.

Stephen C. Redd is a U.S. physician and rear admiral with the U.S. Public Health Service and an Assistant Surgeon General. With over 30 years of public health and executive leadership experience, Redd served as the Director of the Office of Public Health Preparedness and Response at the Centers for Disease Control and Prevention. Previously, he was the Director of the CDC's Influenza Coordination Unit, where he served as the incident commander for the 2009-2010 H1N1 pandemic influenza response.
Daniel R. Lucey is an American physician, researcher, clinical professor of medicine of infectious diseases at Geisel School of Medicine at Dartmouth, and a research associate in anthropology at the Smithsonian National Museum of Natural History, where he has co-organised an exhibition on eight viral outbreaks.
Crystal Watson is a senior scholar at the Johns Hopkins Center for Health Security and an associate professor in the Department of Environmental Health and Engineering. She is an expert in health security, biodefense, and risk assessment and preparedness for emerging infectious diseases. She is currently working on the public health response to the COVID-19 pandemic.