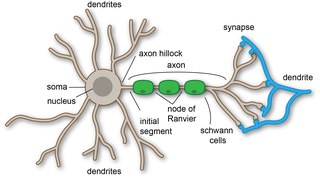
A dendrite or dendron is a branched protoplasmic extension of a nerve cell that propagates the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic tree.

A dendritic spine is a small membranous protrusion from a neuron's dendrite that typically receives input from a single axon at the synapse. Dendritic spines serve as a storage site for synaptic strength and help transmit electrical signals to the neuron's cell body. Most spines have a bulbous head, and a thin neck that connects the head of the spine to the shaft of the dendrite. The dendrites of a single neuron can contain hundreds to thousands of spines. In addition to spines providing an anatomical substrate for memory storage and synaptic transmission, they may also serve to increase the number of possible contacts between neurons. It has also been suggested that changes in the activity of neurons have a positive effect on spine morphology.
In neuroscience, synaptic plasticity is the ability of synapses to strengthen or weaken over time, in response to increases or decreases in their activity. Since memories are postulated to be represented by vastly interconnected neural circuits in the brain, synaptic plasticity is one of the important neurochemical foundations of learning and memory.

Brain-derived neurotrophic factor (BDNF), or abrineurin, is a protein that, in humans, is encoded by the BDNF gene. BDNF is a member of the neurotrophin family of growth factors, which are related to the canonical nerve growth factor (NGF), a family which also includes NT-3 and NT-4/NT-5. Neurotrophic factors are found in the brain and the periphery. BDNF was first isolated from a pig brain in 1982 by Yves-Alain Barde and Hans Thoenen.
Magnocellular neurosecretory cells are large neuroendocrine cells within the supraoptic nucleus and paraventricular nucleus of the hypothalamus. They are also found in smaller numbers in accessory cell groups between these two nuclei, the largest one being the circular nucleus. There are two types of magnocellular neurosecretory cells, oxytocin-producing cells and vasopressin-producing cells, but a small number can produce both hormones. These cells are neuroendocrine neurons, are electrically excitable, and generate action potentials in response to afferent stimulation. Vasopressin is produced from the vasopressin-producing cells via the AVP gene, a molecular output of circadian pathways.

In neuroscience, Golgi cells are the most abundant inhibitory interneurons found within the granular layer of the cerebellum. Golgi cells can be found in the granular layer at various layers. The Golgi cell is essential for controlling the activity of the granular layer. They were first identified as inhibitory in 1964. It was also the first example of an inhibitory feedback network in which the inhibitory interneuron was identified anatomically. Golgi cells produce a wide lateral inhibition that reaches beyond the afferent synaptic field and inhibit granule cells via feedforward and feedback inhibitory loops. These cells synapse onto the dendrite of granule cells and unipolar brush cells. They receive excitatory input from mossy fibres, also synapsing on granule cells, and parallel fibers, which are long granule cell axons. Thereby this circuitry allows for feed-forward and feed-back inhibition of granule cells.

Kalirin, also known as Huntingtin-associated protein-interacting protein (HAPIP), protein duo (DUO), or serine/threonine-protein kinase with Dbl- and pleckstrin homology domain, is a protein that in humans is encoded by the KALRN gene. Kalirin was first identified in 1997 as a protein interacting with huntingtin-associated protein 1. Is also known to play an important role in nerve growth and axonal development.
Michael Greenberg is an American neuroscientist who specializes in molecular neurobiology. He served as the Chair of the Department of Neurobiology at Harvard Medical School from 2008 to 2022.
Activity-dependent plasticity is a form of functional and structural neuroplasticity that arises from the use of cognitive functions and personal experience; hence, it is the biological basis for learning and the formation of new memories. Activity-dependent plasticity is a form of neuroplasticity that arises from intrinsic or endogenous activity, as opposed to forms of neuroplasticity that arise from extrinsic or exogenous factors, such as electrical brain stimulation- or drug-induced neuroplasticity. The brain's ability to remodel itself forms the basis of the brain's capacity to retain memories, improve motor function, and enhance comprehension and speech amongst other things. It is this trait to retain and form memories that is associated with neural plasticity and therefore many of the functions individuals perform on a daily basis. This plasticity occurs as a result of changes in gene expression which are triggered by signaling cascades that are activated by various signaling molecules during increased neuronal activity.

Activity-regulated cytoskeleton-associated protein is a plasticity protein that in humans is encoded by the ARC gene. The gene is believed to derive from a retrotransposon. The protein is found in the neurons of tetrapods and other animals where it can form virus-like capsids that transport RNA between neurons.
Synaptic tagging, or the synaptic tagging hypothesis, was first proposed in 1997 by Julietta U. Frey and Richard G. Morris; it seeks to explain how neural signaling at a particular synapse creates a target for subsequent plasticity-related product (PRP) trafficking essential for sustained LTP and LTD. Although the molecular identity of the tags remains unknown, it has been established that they form as a result of high or low frequency stimulation, interact with incoming PRPs, and have a limited lifespan.
Addiction is a state characterized by compulsive engagement in rewarding stimuli, despite adverse consequences. The process of developing an addiction occurs through instrumental learning, which is otherwise known as operant conditioning.
James O. McNamara is an American neurologist and neuroscientist, known for his research of epileptogenesis, the process underlying development and progression of epilepsy. He is the Duke School of Medicine Professor of Neuroscience in the Departments of Neurobiology, Neurology, and Pharmacology and Cancer Biology at Duke University. He served as chair of the Department of Neurobiology at Duke from 2002 to 2011

Synaptic stabilization is crucial in the developing and adult nervous systems and is considered a result of the late phase of long-term potentiation (LTP). The mechanism involves strengthening and maintaining active synapses through increased expression of cytoskeletal and extracellular matrix elements and postsynaptic scaffold proteins, while pruning less active ones. For example, cell adhesion molecules (CAMs) play a large role in synaptic maintenance and stabilization. Gerald Edelman discovered CAMs and studied their function during development, which showed CAMs are required for cell migration and the formation of the entire nervous system. In the adult nervous system, CAMs play an integral role in synaptic plasticity relating to learning and memory.
Catherine S. Woolley is an American neuroendocrinologist. Woolley holds the William Deering Chair in Biological Sciences in the Department of Neurobiology, Weinberg College of Arts & Sciences, at Northwestern University. She is also a member of the Women's Health Research Institute in the Feinberg School of Medicine at Northwestern University.
HollisT. Cline is an American neuroscientist and the Director of the Dorris Neuroscience Center at the Scripps Research Institute in California. Her research focuses on the impact of sensory experience on brain development and plasticity.

Brenda Bloodgood is an American neuroscientist and associate professor of neurobiology at the University of California, San Diego. Bloodgood studies the molecular and cellular basis of brain circuitry changes in response to an animal's interactions with the environment.
David E. Olson is an American chemist and neuroscientist. He is an associate professor of chemistry, biochemistry and molecular medicine at the University of California, Davis, and is the founding director of the UC Davis Institute for Psychedelics and Neurotherapeutics.
Saak Victor Ovsepian is an Armenian-Irish neuroscientist best known for his research in neurobiology, neurotherapeutics and translational biosciences. He is a professor in biosciences at the University of Greenwich.

Morgan Hwa-Tze Sheng is a professor of neurobiology and a Core Institute Member at the Broad Institute, where he is a co-director of the Stanley Center for Psychiatric Research at Broad Institute. He is a professor of neuroscience in the Department of Brain and Cognitive Sciences as well as the Menicon Professor of Neuroscience at Massachusetts Institute of Technology. He is also an associate member at both The Picower Institute for Learning and McGovern Institute for Brain Research. He has served on the editorial boards of Current Opinions in Neurobiology, Neuron, and The Journal of Neuroscience.








