
Emergency contraception (EC) is a birth control measure, used after sexual intercourse to prevent pregnancy.

Medicare is a government national health insurance program in the United States, begun in 1965 under the Social Security Administration (SSA) and now administered by the Centers for Medicare and Medicaid Services (CMS). It primarily provides health insurance for Americans aged 65 and older, but also for some younger people with disability status as determined by the SSA, including people with end stage renal disease and amyotrophic lateral sclerosis.

The Centers for Medicare & Medicaid Services (CMS), is a federal agency within the United States Department of Health and Human Services (HHS) that administers the Medicare program and works in partnership with state governments to administer Medicaid, the Children's Health Insurance Program (CHIP), and health insurance portability standards. In addition to these programs, CMS has other responsibilities, including the administrative simplification standards from the Health Insurance Portability and Accountability Act of 1996 (HIPAA), quality standards in long-term care facilities through its survey and certification process, clinical laboratory quality standards under the Clinical Laboratory Improvement Amendments, and oversight of HealthCare.gov. CMS was previously known as the Health Care Financing Administration (HCFA) until 2001.

Glipizide, sold under the brand name Glucotrol among others, is an anti-diabetic medication of the sulfonylurea class used to treat type 2 diabetes. It is used together with a diabetic diet and exercise. It is not indicated for use by itself in type 1 diabetes. It is taken by mouth. Effects generally begin within half an hour and can last for up to a day.
Prescription drug list prices in the United States continually rank among the highest in the world. The high cost of prescription drugs became a major topic of discussion in the 21st century, leading up to the U.S. health care reform debate of 2009, and received renewed attention in 2015. One major reason for high prescription drug prices in the United States relative to other countries is the inability of government-granted monopolies in the U.S. health care sector to use their bargaining power to negotiate lower prices and that the US payer ends up subsidizing the world's R&D spending on drugs. The Democratic Party is broadly in favor of allowing the government to negotiate drug prices, whereas the Republican Party has prevented passage of bills that would permit that.

Naproxen, sold under the brand name Aleve or Apronax among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain, menstrual cramps, inflammatory diseases such as rheumatoid arthritis, gout and fever. It is taken orally. It is available in immediate and delayed release formulations. Onset of effects is within an hour and last for up to twelve hours.
The pregnancy category of a medication is an assessment of the risk of fetal injury due to the pharmaceutical, if it is used as directed by the mother during pregnancy. It does not include any risks conferred by pharmaceutical agents or their metabolites in breast milk.

Rosuvastatin, sold under the brand name Crestor among others, is a statin medication, used to prevent cardiovascular disease in those at high risk and treat abnormal lipids. It is recommended to be used together with dietary changes, exercise, and weight loss. It is taken by mouth.
The United States Pharmacopeia (USP) is a pharmacopeia for the United States published annually by the United States Pharmacopeial Convention, a nonprofit organization that owns the trademark and also owns the copyright on the pharmacopeia itself. The USP is published in a combined volume with the National Formulary as the USP-NF. If a drug ingredient or drug product has an applicable USP quality standard, it must conform in order to use the designation "USP" or "NF". Drugs subject to USP standards include both human drugs and animal drugs. USP-NF standards also have a role in US federal law; a drug or drug ingredient with a name recognized in USP-NF is considered adulterated if it does not satisfy compendial standards for strength, quality or purity. USP also sets standards for dietary supplements and food ingredients. USP has no role in enforcing its standards; enforcement is the responsibility of the U.S. Food and Drug Administration (FDA) and other government authorities in the United States.

Dicycloverine, also known as dicyclomine, sold under the brand name Bentyl in the US, is a medication that is used to treat spasms of the intestines such as occur in irritable bowel syndrome. It is taken by mouth or by injection into a muscle. While it has been used in baby colic and enterocolitis, evidence does not support these uses.
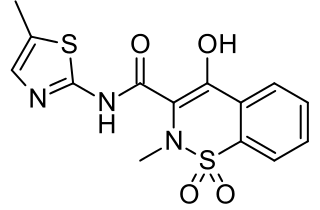
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammation in rheumatic diseases and osteoarthritis. It is used by mouth or by injection into a vein. It is recommended that it be used for as short a period as possible and at a low dose.

Capecitabine, sold under the brand name Xeloda among others, is a chemotherapy medication used to treat breast cancer, gastric cancer and colorectal cancer. For breast cancer it is often used together with docetaxel. It is taken by mouth.

Medicare Part D, also called the Medicare prescription drug benefit, is an optional United States federal-government program to help Medicare beneficiaries pay for self-administered prescription drugs. Part D was enacted as part of the Medicare Modernization Act of 2003 and went into effect on January 1, 2006. Under the program, drug benefits are provided by private insurance plans that receive premiums from both enrollees and the government. Part D plans typically pay most of the cost for prescriptions filled by their enrollees. However, plans are later reimbursed for much of this cost through rebates paid by manufacturers and pharmacies.

AIDS Healthcare Foundation (AHF) is a Los Angeles-based 501(c)(3) nonprofit organization providing medicine and health care to individuals living with or affected by HIV/AIDS. As of 2020, it operates 64 outpatient healthcare centers and 48 pharmacies in 15 states.
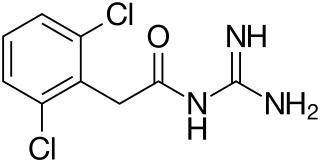
Guanfacine, sold under the brand name Tenex among others, is an oral medication used to treat attention deficit hyperactivity disorder (ADHD) and high blood pressure. It is not considered a first-line treatment for either indication.

Travoprost, sold under the brand name Travatan among others, is a medication used to treat high pressure inside the eye including glaucoma. Specifically it is used for open angle glaucoma when other agents are not sufficient. It is used as an eye drop. Effects generally occur within two hours.
Pharmaceutical policy is a branch of health policy that deals with the development, provision and use of medications within a health care system. It embraces drugs, biologics, vaccines and natural health products.
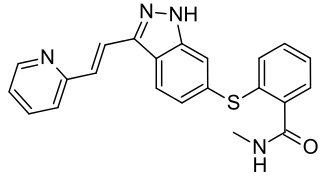
Axitinib, sold under the brand name Inlyta, is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.
Pharmaceutical fraud involves activities that result in false claims to insurers or programs such as Medicare in the United States or equivalent state programs for financial gain to a pharmaceutical company. There are several different schemes used to defraud the health care system which are particular to the pharmaceutical industry. These include: Good Manufacturing Practice (GMP) Violations, Off Label Marketing, Best Price Fraud, CME Fraud, Medicaid Price Reporting, and Manufactured Compound Drugs. Examples of fraud cases include the GlaxoSmithKline $3 billion settlement, Pfizer $2.3 billion settlement, and Merck $650 million settlement. Damages from fraud can be recovered by use of the False Claims Act, most commonly under the qui tam provisions which rewards an individual for being a "whistleblower", or relator (law).
The Affordable Care Act (ACA) is divided into 10 titles and contains provisions that became effective immediately, 90 days after enactment, and six months after enactment, as well as provisions phased in through to 2020. Below are some of the key provisions of the ACA. For simplicity, the amendments in the Health Care and Education Reconciliation Act of 2010 are integrated into this timeline.











