Related Research Articles

Adenosine triphosphate (ATP) is an organic compound and hydrotrope that provides energy to drive many processes in living cells, e.g. muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP so that the human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme.
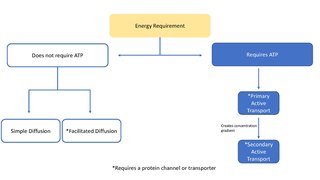
In cellular biology, active transport is the movement of molecules across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate (ATP), and secondary active transport that uses an electrochemical gradient. An example of active transport in human physiology is the uptake of glucose in the intestines.
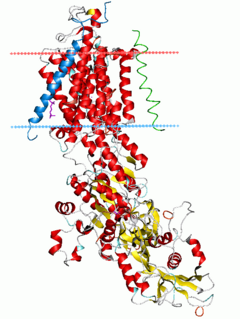
Na⁺/K⁺-ATPase is an enzyme found in the membrane of all animal cells. It performs several functions in cell physiology.
A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane protein; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion or active transport. The two main types of proteins involved in such transport are broadly categorized as either channels or carriers. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans. Collectively membrane transporters and channels are transportome. Transportomes govern cellular influx and efflux of not only ions and nutrients but drugs as well.

The ATP-binding cassette transporters are a transport system superfamily that is one of the largest and possibly one of the oldest gene families. It is represented in all extant phyla, from prokaryotes to humans.

P-glycoprotein 1 also known as multidrug resistance protein 1 (MDR1) or ATP-binding cassette sub-family B member 1 (ABCB1) or cluster of differentiation 243 (CD243) is an important protein of the cell membrane that pumps many foreign substances out of cells. More formally, it is an ATP-dependent efflux pump with broad substrate specificity. It exists in animals, fungi, and bacteria, and it likely evolved as a defense mechanism against harmful substances.
Dephosphorylation is the removal of a phosphate (PO43−) group from an organic compound by hydrolysis. It is a reversible post-translational modification. Dephosphorylation and its counterpart, phosphorylation, activate and deactivate enzymes by detaching or attaching phosphoric esters and anhydrides. A notable occurrence of dephosphorylation is the conversion of ATP to ADP and inorganic phosphate.

The bafilomycins are a family of macrolide antibiotics produced from a variety of Streptomycetes. Their chemical structure is defined by a 16-membered lactone ring scaffold. Bafilomycins exhibit a wide range of biological activity, including anti-tumor, anti-parasitic, immunosuppressant and anti-fungal activity. The most used bafilomycin is bafilomycin A1, a potent inhibitor of cellular autophagy. Bafilomycins have also been found to act as ionophores, transporting potassium K+ across biological membranes and leading to mitochondrial damage and cell death.
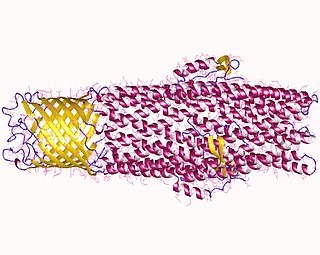
In biology, a transporter is a transmembrane protein that moves ions across a biological membrane to accomplish many different biological functions including, cellular communication, maintaining homeostasis, energy production, ect. There are different types of transporters including, pumps, uniporters, antiporters, and symporters. Active transporters or ion pumps are transporters that convert energy from various sources—including adenosine triphosphate (ATP), sunlight, and other redox reactions—to potential energy by pumping an ion up its concentration gradient. This potential energy could then used by secondary transporters, including ion carriers and ion channels, to drive vital cellular processes, such as ATP synthesis.
All microorganisms, with a few exceptions, have highly conserved DNA sequences in their genome that are transcribed and translated to efflux pumps. Efflux pumps are capable of moving a variety of different toxic compounds out of cells, such as antibiotics, heavy metals, organic pollutants, plant-produced compounds, quorum sensing signals, bacterial metabolites and neurotransmitters via active efflux, which is vital part for xenobiotic metabolism. This active efflux mechanism is responsible for various types of resistance to bacterial pathogens within bacterial species - the most concerning being antibiotic resistance because microorganisms can have adapted efflux pumps to divert toxins out of the cytoplasm and into extracellular media.
Calcein, also known as fluorexon, fluorescein complex, is a fluorescent dye with excitation and emission wavelengths of 495/515 nm, respectively, and has the appearance of orange crystals. Calcein self-quenches at concentrations above 70mM and is commonly used as an indicator of lipid vesicle leakage. It is also used traditionally as a complexometric indicator for titration of calcium ions with EDTA, and for fluorometric determination of calcium.
Neurotransmitter transporters are a class of membrane transport proteins that span the cellular membranes of neurons. Their primary function is to carry neurotransmitters across these membranes and to direct their further transport to specific intracellular locations. There are more than twenty types of neurotransmitter transporters.
Translocase is a general term for a protein that assists in moving another molecule, usually across a cell membrane. These enzymes catalyze the movement of ions or molecules across membranes or their separation within membranes. The reaction is designated as a transfer from “side 1” to “side 2” because the designations “in” and “out”, which had previously been used, can be ambiguous. Translocases are the most common secretion system in Gram positive bacteria.

Multidrug resistance-associated protein 1 (MRP1) is a protein that in humans is encoded by the ABCC1 gene.
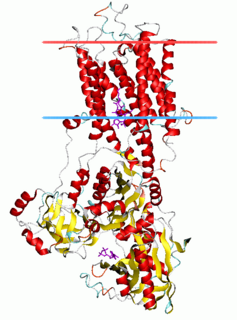
The P-type ATPases, also known as E1-E2 ATPases, are a large group of evolutionarily related ion and lipid pumps that are found in bacteria, archaea, and eukaryotes. P-type ATPases are α-helical bundle primary transporters named based upon their ability to catalyze auto- (or self-) phosphorylation (hence P) of a key conserved aspartate residue within the pump and their energy source, adenosine triphosphate (ATP). In addition, they all appear to interconvert between at least two different conformations, denoted by E1 and E2. P-type ATPases fall under the P-type ATPase (P-ATPase) Superfamily (TC# 3.A.3) which, as of early 2016, includes 20 different protein families.

Multidrug resistance-associated protein 2 (MRP2) also called canalicular multispecific organic anion transporter 1 (cMOAT) or ATP-binding cassette sub-family C member 2 (ABCC2) is a protein that in humans is encoded by the ABCC2 gene.
Arsenite-antimonite transporters are membrane transporters that pump arsenite or antimonite out of a cell. Antimonite is the salt of antimony and has been found to significantly impact the toxicity of arsenite. The similar structure of As(III) and Sb(III) makes it plausible that certain transporters function in the efflux of both substrates. Arsenic efflux transporters exist in almost every organism and serve to remove this toxic compound from the cell.
Multidrug resistance pumps also known Multidrug efflux pumps are a type of efflux pump and P-glycoprotein. MDR pumps in the cell membrane extrudes many foreign substances out of the cells and some pumps can have a broad specificity. MDR pumps exist in animals, fungi, and bacteria and likely evolved as a defense mechanism against harmful substances. There are seven families of MDRs and are grouped by homology, energy source, and overall structure.

Resistance-nodulation-division (RND) family transporters are a category of bacterial efflux pumps, especially identified in Gram-negative bacteria and located in the cytoplasmic membrane, that actively transport substrates. The RND superfamily includes seven families: the heavy metal efflux (HME), the hydrophobe/amphiphile efflux-1, the nodulation factor exporter family (NFE), the SecDF protein-secretion accessory protein family, the hydrophobe/amphiphile efflux-2 family, the eukaryotic sterol homeostasis family, and the hydrophobe/amphiphile efflux-3 family. These RND systems are involved in maintaining homeostasis of the cell, removal of toxic compounds, and export of virulence determinants. They have a broad substrate spectrum and can lead to the diminished activity of unrelated drug classes if over-expressed. The first reports of drug resistant bacterial infections were reported in the 1940s after the first mass production of antibiotics. Most of the RND superfamily transport systems are made of large polypeptide chains. RND proteins exist primarily in gram-negative bacteria but can also be found in gram-positive bacteria, archaea, and eukaryotes.

Wilfred D. Stein is a writer and biophysicist who has applied mathematical principles to medical, biologic, and oncologic problems.
References
- ↑ Glavinas H, Méhn D, Jani M, Oosterhuis B, Herédi-Szabó K, Krajcsi P (June 2008). "Utilization of membrane vesicle preparations to study drug-ABC transporter interactions". Expert Opinion on Drug Metabolism & Toxicology. 4 (6): 721–32. doi:10.1517/17425255.4.6.721. PMID 18611113.
- ↑ Kis E, Rajnai Z, Ioja E, et al. (January 2009). "Mouse Bsep ATPase assay: a nonradioactive tool for assessment of the cholestatic potential of drugs". Journal of Biomolecular Screening. 14 (1): 10–5. doi: 10.1177/1087057108326145 . PMID 19029016.
- ↑ Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA (March 1992). "Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase". The Journal of Biological Chemistry. 267 (7): 4854–8. PMID 1347044.