
Over-the-counter (OTC) drugs are medicines sold directly to a consumer without a requirement for a prescription from a healthcare professional, as opposed to prescription drugs, which may be supplied only to consumers possessing a valid prescription. In many countries, OTC drugs are selected by a regulatory agency to ensure that they contain ingredients that are safe and effective when used without a physician's care. OTC drugs are usually regulated according to their active pharmaceutical ingredient (API) rather than final products. By regulating APIs instead of specific drug formulations, governments allow manufacturers the freedom to formulate ingredients, or combinations of ingredients, into proprietary mixtures.

Zolpidem, sold under the brand name Ambien, among others, is a medication primarily used for the short-term treatment of sleeping problems. Guidelines recommend that it be used only after cognitive behavioral therapy for insomnia and behavioral changes, such as sleep hygiene, have been tried. It decreases the time to sleep onset by about fifteen minutes and at larger doses helps people stay asleep longer. It is taken by mouth and is available in conventional tablets, sublingual tablets, or oral spray.

A prescription drug is a pharmaceutical drug that legally requires a medical prescription to be dispensed. In contrast, over-the-counter drugs can be obtained without a prescription. The reason for this difference in substance control is the potential scope of misuse, from drug abuse to practicing medicine without a license and without sufficient education. Different jurisdictions have different definitions of what constitutes a prescription drug.
The pregnancy category of a medication is an assessment of the risk of fetal injury due to the pharmaceutical, if it is used as directed by the mother during pregnancy. It does not include any risks conferred by pharmaceutical agents or their metabolites in breast milk.
The Pharmaceutical Benefits Scheme (PBS) is a program of the Australian Government that subsidises prescription medication for Australian citizens and permanent residents, as well as international visitors covered by a reciprocal health care agreement. The PBS is separate to the Medicare Benefits Schedule, a list of health care services that can be claimed under Medicare, Australia's universal health care insurance scheme.

Dextropropoxyphene is an analgesic in the opioid category, patented in 1955 and manufactured by Eli Lilly and Company. It is an optical isomer of levopropoxyphene. It is intended to treat mild pain and also has antitussive and local anaesthetic effects. The drug has been taken off the market in Europe and the US due to concerns of fatal overdoses and heart arrhythmias. It is still available in Australia, albeit with restrictions after an application by its manufacturer to review its proposed banning. Its onset of analgesia is said to be 20–30 minutes and peak effects are seen about 1.5–2.0 hours after oral administration.

The regulation of therapeutic goods, defined as drugs and therapeutic devices, varies by jurisdiction. In some countries, such as the United States, they are regulated at the national level by a single agency. In other jurisdictions they are regulated at the state level, or at both state and national levels by various bodies, as in Australia.
Pharmacovigilance, also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: pharmakon and vigilare. As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy. Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
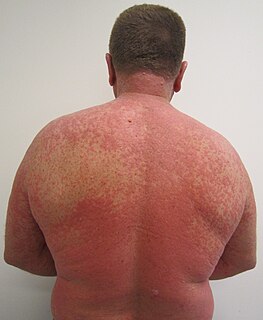
An adverse drug reaction (ADR) is a harmful, unintended result caused by taking medication. ADRs may occur following a single dose or prolonged administration of a drug or result from the combination of two or more drugs. The meaning of this term differs from the term "side effect" because side effects can be beneficial as well as detrimental. The study of ADRs is the concern of the field known as pharmacovigilance. An adverse drug event (ADE) refers to any unexpected and inappropriate occurrence at the time a drug is used, whether or not associated with the administration of the drug. An ADR is a special type of ADE in which a causative relationship can be shown. ADRs are only one type of medication-related harm, as harm can also be caused by omitting to take indicated medications.

Lumiracoxib is a COX-2 selective inhibitor nonsteroidal anti-inflammatory drug.
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. If it results from an unsuitable or incorrect dosage or procedure, this is called a medical error and not a complication. Adverse effects are sometimes referred to as "iatrogenic" because they are generated by a physician/treatment. Some adverse effects occur only when starting, increasing or discontinuing a treatment. Adverse effects can also be caused by placebo treatments . Using a drug or other medical intervention which is contraindicated may increase the risk of adverse effects. Adverse effects may cause complications of a disease or procedure and negatively affect its prognosis. They may also lead to non-compliance with a treatment regimen. Adverse effects of medical treatment resulted in 142,000 deaths in 2013 up from 94,000 deaths in 1990 globally.
Established in March 1998, NPS MedicineWise is an Australian not-for-profit organisation whose programs are funded by the national Department of Health. Since July 2012, the organisation has been officially known as NPS MedicineWise.
The Australian Drug Evaluation Committee or ADEC, was a committee that provided independent scientific advice to the Australian Government regarding therapeutic drugs. The committee was originally formed in 1963 and more recently authorised under the Therapeutic Goods Act 1989 (Cth) as part of the Therapeutic Goods Administration (TGA). In 2010, ADEC was replaced by the Advisory Committee on Prescription Medicines (ACPM)
The Standard for the Uniform Scheduling of Medicines and Poisons (SUSMP) is an Australian legislative instrument produced by the Therapeutic Goods Administration (TGA). Before 2010, it was known as the Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP). The SUSMP classifies drugs and poisons into different Schedules signifying the degree of control recommended to be exercised over their availability to the public. As of 2022, the most recent version is the Poisons Standard February 2022.
A patient safety organization (pso) is a group, institution, or association that improves medical care by reducing medical errors. Common functions of patient safety organizations are data collection and analysis, reporting, education, funding, and advocacy. A pso differs from a Federally designed Patient Safety Organization (PSO), which provides health care providers in the U.S. privilege and confidentiality protections for efforts to improve patient safety and the quality of patient care delivery
At its most basic level, a formulary is a list of medicines. Traditionally, a formulary contained a collection of formulas for the compounding and testing of medication. Today, the main function of a prescription formulary is to specify particular medications that are approved to be prescribed at a particular hospital, in a particular health system, or under a particular health insurance policy. The development of prescription formularies is based on evaluations of efficacy, safety, and cost-effectiveness of drugs.
The following outline is provided as an overview of and topical guide to clinical research:
Electronic prescription is the computer-based electronic generation, transmission, and filling of a medical prescription, taking the place of paper and faxed prescriptions. E-prescribing allows a physician, physician assistant, pharmacist, or nurse practitioner to use digital prescription software to electronically transmit a new prescription or renewal authorization to a community or mail-order pharmacy. It outlines the ability to send error-free, accurate, and understandable prescriptions electronically from the healthcare provider to the pharmacy. E-prescribing is meant to reduce the risks associated with traditional prescription script writing. It is also one of the major reasons for the push for electronic medical records. By sharing medical prescription information, e-prescribing seeks to connect the patient's team of healthcare providers to facilitate knowledgeable decision making.
Cemiplimab, sold under the brand name Libtayo, is a monoclonal antibody medication for the treatment of squamous cell skin cancer. Cemiplimab belongs to a class of drugs that binds to the programmed death receptor-1 (PD-1), blocking the PD-1/PD-L1 pathway.

Drug utilization review refers to a review of prescribing, dispensing, administering and ingesting of medication. This authorized, structured and ongoing review is related to pharmacy benefit managers. Drug use/ utilization evaluation and medication utilization evaluations are the same as drug utilization review.







