Related Research Articles

Chemotherapy is a type of cancer treatment that uses one or more anti-cancer drugs as part of a standardized chemotherapy regimen. Chemotherapy may be given with a curative intent, or it may aim to prolong life or to reduce symptoms. Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called medical oncology.
A tranquilizer refers to a drug which is designed for the treatment of anxiety, fear, tension, agitation, and disturbances of the mind, specifically to reduce states of anxiety and tension.

In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.

Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a group of pharmaceuticals that are used to suppress abnormal rhythms of the heart, such as atrial fibrillation, atrial flutter, ventricular tachycardia, and ventricular fibrillation.
Protease inhibitors (PIs) are a class of antiviral drugs that are widely used to treat HIV/AIDS and hepatitis C. Protease inhibitors prevent viral replication by selectively binding to viral proteases and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles.
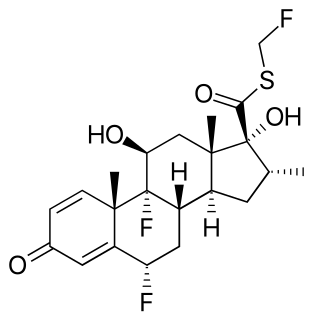
Fluticasone are two manufactured steroids used to treat nasal symptoms. Both the esters, fluticasone furoate and fluticasone propionate, are also used as topical anti-inflammatories and inhaled corticosteroids, and are used much more commonly in comparison.
A British Approved Name (BAN) is the official, non-proprietary, or generic name given to a pharmaceutical substance, as defined in the British Pharmacopoeia (BP).
The BAN is also the official name used in some countries across the world, because starting in 1953, proposed new names were evaluated by a panel of experts from WHO in conjunction with the BP commission to ensure naming consistency worldwide. There is also a British Approved Name (Modified) (BANM).

Ethosuximide, sold under the brand name Zarontin among others, is a medication used to treat absence seizures. It may be used by itself or with other antiseizure medications such as valproic acid. Ethosuximide is taken by mouth.

A lotion is a low-viscosity topical preparation intended for application to the skin. By contrast, creams and gels have higher viscosity, typically due to lower water content. Lotions are applied to external skin with bare hands, a brush, a clean cloth, or cotton wool.
Antiprotozoal agents is a class of pharmaceuticals used in treatment of protozoan infection.
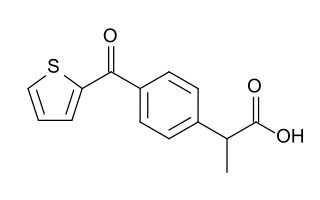
Suprofen is a nonsteroidal anti-inflammatory drug (NSAID) developed by Janssen Pharmaceutica that was marketed as 1% eye drops under the trade name Profenal.

Tuaminoheptane is a sympathomimetic agent and vasoconstrictor which was formerly used as a nasal decongestant. It has also been used as a stimulant.
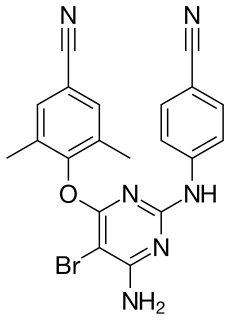
Etravirine is a drug used for the treatment of HIV. Etravirine is a non-nucleoside reverse transcriptase inhibitor (NNRTI). Unlike the currently available agents in the class, resistance to other NNRTIs does not seem to confer resistance to etravirine. Etravirine is marketed by Tibotec, a subsidiary of Johnson & Johnson. In January 2008, the Food and Drug Administration approved its use for patients with established resistance to other drugs, making it the 30th anti-HIV drug approved in the United States and the first to be approved in 2008. It was also approved for use in Canada on April 1, 2008.

The Philippine Drug Enforcement Agency is the lead anti-drug law enforcement agency, responsible for preventing, investigating and combating any dangerous drugs, controlled precursors and essential chemicals within the Philippines. The agency is tasked with the enforcement of the penal and regulatory provisions of Republic Act No. 9165, otherwise known as the Comprehensive Dangerous Drugs Act of 2002.

Thin-film drug delivery uses a dissolving film or oral drug strip to administer drugs via absorption in the mouth and/or via the small intestines (enterically). A film is prepared using hydrophilic polymers that rapidly dissolves on the tongue or buccal cavity, delivering the drug to the systemic circulation via dissolution when contact with liquid is made.

Cyclofenil, sold under the brand name Sexovid among others, is a selective estrogen receptor modulator (SERM) medication which is used as a gonadotropin stimulant or ovulation inducer and in menopausal hormone therapy in women. It is mostly no longer available. The medication is taken by mouth.
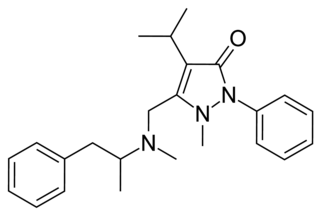
Famprofazone is a nonsteroidal anti-inflammatory agent (NSAID) of the pyrazolone series which is available over-the-counter in some countries such as Taiwan. It has analgesic, anti-inflammatory, and antipyretic effects. Famprofazone has been known to produce methamphetamine as an active metabolite, with 15-20% of an oral dose being converted to it. As a result, famprofazone has occasionally been implicated in causing positives on drug tests for amphetamines.

Rosonabant (INN; E-6776) is a drug acting as a CB1 receptor antagonist/inverse agonist that was under investigation by Esteve as an appetite suppressant for the treatment of obesity. Development of the drug for clinical use was apparently halted shortly after the related CB1 antagonist rimonabant was discontinued, likely due to the reports of severe psychiatric adverse effects such as anxiety, depression, and suicidal ideation associated with it and with similarly-acting agents.
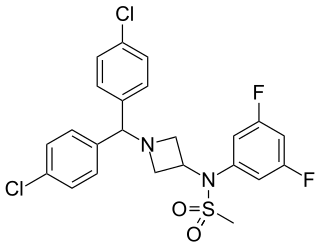
Drinabant (INN; AVE-1625) is a drug that acts as a selective CB1 receptor antagonist, which was under investigation varyingly by Sanofi-Aventis as a treatment for obesity, schizophrenia, Alzheimer's disease, Parkinson's disease, and nicotine dependence. Though initially studied as a potential treatment for a variety of different medical conditions, Sanofi-Aventis eventually narrowed down the therapeutic indications of the compound to just appetite suppression. Drinabant reached phase IIb clinical trials for this purpose in the treatment of obesity but was shortly thereafter discontinued, likely due to the observation of severe psychiatric side effects including anxiety, depression, and thoughts of suicide in patients treated with the now-withdrawn rimonabant, another CB1 antagonist that was also under development by Sanofi-Aventis.

Oxabolone is a synthetic anabolic-androgenic steroid (AAS) of the nandrolone (19-nortestosterone) group which was never marketed. It can be formulated as the cipionate ester prodrug oxabolone cipionate, which, in contrast, has been marketed for medical use.
References
| This biochemistry article is a stub. You can help Wikipedia by expanding it. |