
A dietary supplement is a manufactured product intended to supplement a person's diet by taking a pill, capsule, tablet, powder, or liquid. A supplement can provide nutrients either extracted from food sources, or that are synthetic. The classes of nutrient compounds in supplements include vitamins, minerals, fiber, fatty acids, and amino acids. Dietary supplements can also contain substances that have not been confirmed as being essential to life, and so are not nutrients per se, but are marketed as having a beneficial biological effect, such as plant pigments or polyphenols. Animals can also be a source of supplement ingredients, such as collagen from chickens or fish for example. These are also sold individually and in combination, and may be combined with nutrient ingredients. The European Commission has also established harmonized rules to help insure that food supplements are safe and appropriately labeled.

Novartis AG is a Swiss multinational pharmaceutical corporation based in Basel, Switzerland. Consistently ranked in the global top five, Novartis is one of the largest pharmaceutical companies in the world and was the fourth largest by revenue in 2022.
In the U.S. and Canada, the Reference Daily Intake (RDI) is used in nutrition labeling on food and dietary supplement products to indicate the daily intake level of a nutrient that is considered to be sufficient to meet the requirements of 97–98% of healthy individuals in every demographic in the United States. While developed for the US population, it has been adopted by other countries, such as Canada.

Olestra is a fat substitute that adds no metabolizable calories to products. It has been used in the preparation of otherwise high-fat foods, thereby lowering or eliminating their fat content. The Food and Drug Administration (FDA) originally approved olestra for use in the US as a replacement for fats and oils in prepackaged ready-to-eat snacks in 1996, concluding that such use "meets the safety standard for food additives, reasonable certainty of no harm". In the late 2000s, olestra lost its popularity due to supposed side effects and has been largely phased out, but products containing the ingredient can still be purchased at grocery stores in some countries. As of 2024, no products are sold in the United States using Olestra.

A multivitamin is a preparation intended to serve as a dietary supplement with vitamins, dietary minerals, and other nutritional elements. Such preparations are available in the form of tablets, capsules, pastilles, powders, liquids, or injectable formulations. Other than injectable formulations, which are only available and administered under medical supervision, multivitamins are recognized by the Codex Alimentarius Commission as a category of food.
Nutraceutical is a marketing term used to imply a pharmaceutical effect from a compound or food product that has not been scientifically confirmed or approved to have clinical benefits. In the United States, nutraceuticals are unregulated, existing in the same category as dietary supplements and food additives by the Food and Drug Administration (FDA), under the authority of the Federal Food, Drug, and Cosmetic Act.

GNC Holdings, LLC is an American multinational retail and nutritional manufacturing company based in Pittsburgh, Pennsylvania. It specializes in health and nutrition related products, including vitamins, supplements, minerals, herbs, sports nutrition, diet, and energy products.

The nutrition facts label is a label required on most packaged food in many countries, showing what nutrients and other ingredients are in the food. Labels are usually based on official nutritional rating systems. Most countries also release overall nutrition guides for general educational purposes. In some cases, the guides are based on different dietary targets for various nutrients than the labels on specific foods.
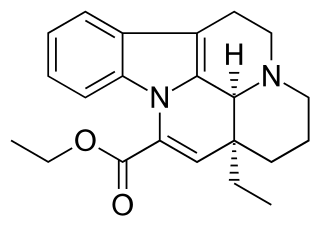
Vinpocetine is a synthetic derivative of the vinca alkaloid vincamine, differing by the removal of a hydroxyl group and by being the ethyl rather than the methyl ester of the underlying carboxylic acid. Vincamine is extracted from either the seeds of Voacanga africana or the leaves of Vinca minor.
Hydroxycut is a brand of dietary supplements that is marketed as a weight loss aid. Hydroxycut was originally developed and manufactured by MuscleTech Research and Development; MuscleTech was sold to Iovate Health Sciences in 2003–2004 and declared bankruptcy in 2005; Iovate continues to use MuscleTech as a brand to market Hydroxycut.
Cold Capsule IV and Cold Capsule V were extended release oral capsules (pill) used to control cold symptoms.
The Center for Food Safety and Applied Nutrition is the branch of the United States Food and Drug Administration (FDA) that regulates food, dietary supplements, and cosmetics, as opposed to drugs, biologics, medical devices, and radiological products, which also fall under the purview of the FDA.
Dexatrim is an over-the-counter (OTC) dietary supplement meant to assist with weight loss. Dexatrim claims it "gives you the power to lose weight, curb binges, and keep you in control of your diet." Current Dexatrim products available are in capsule form and include Dexatrim Max Complex 7, Dexatrim Max Daytime Appetite Control, Dexatrim Natural Green Tea, and Dexatrim Natural Extra Energy. The major active ingredients found in current Dexatrim products include caffeine, green tea extract, Asian (Panax) ginseng root extract, and dehydroepiandrosterone (DHEA).
ConsumerLab.com, LLC. is a privately held American company registered in White Plains, NY. It is a publisher of test results on health, wellness, and nutrition products. Consumer Labs is not a laboratory, but contracts studies to outside testing laboratories. It purchases dietary supplement products and other consumer goods directly from public storefronts and online retailers, contracts for testing by private laboratories, and publishes reports based on the results. It primarily derives revenue from the sale of subscriptions to its online publications, which are paywalled. Other sources of revenue include a proprietary certification program, licensing fees, contents re-publication license fees, and advertising.

The Nutrition Labeling and Education Act (NLEA) is a 1990 United States Federal law. It was signed into law on November 8, 1990 by President George H. W. Bush.

Swanson Health Products (SHP) is a natural health catalog and Internet marketing company headquartered in Fargo, North Dakota. The company sells natural health and wellness products, including health foods, dietary supplements such as vitamins, minerals, herbs, as well as natural personal care products directly to consumers through mail-order catalogs and an e-commerce website.

The Dietary Supplement Health and Education Act of 1994 ("DSHEA"), is a 1994 statute of United States Federal legislation which defines and regulates dietary supplements. Under the act, supplements are regulated by the FDA for Good Manufacturing Practices under 21 CFR Part 111. The act was intended to exempt the dietary and herbal supplement industry from most FDA drug regulations, allowing them to be sold and marketed without scientific backing for their health and medical claims.
Pre-workout is a generic term for a range of bodybuilding supplement products used by athletes and weightlifters to enhance athletic performance. Supplements are taken to increase endurance, energy, and focus during a workout. Pre-workout supplements contain a variety of ingredients such as caffeine and creatine, differing by capsule or powder products. The first pre-workout product entered the market in 1982, and since then the category has grown in use. Some pre-workout products contain ingredients linked to adverse effects. Although these products are not regulated, the Food and Drug Administration (FDA) warns consumers to be cautious when consuming them.

The Proxmire Amendments were a series of legislation that prohibited the Food and Drug Administration from monitoring and limiting the potency of vitamins and minerals found in dietary supplements. The Proxmire Amendment also made it so that food supplements could not be classified as drugs, making their sale possible without a prescription from a doctor. According to a study done, "dietary supplements fall into the following categories: vitamins, minerals, herbs or other botanicals, amino acids, animal-derived products, hormones and hormone analogues, enzymes, and concentrates, metabolites, constituents, or extracts of these." They can be used by anyone wishing to purchase them as much or as little as they desire. Dietary supplements can be used to increase productivity, treat illness, help mental health such as depression and anxiety, enhancing mental abilities, building muscle, or losing weight, among many other uses. William Proxmire, a Senator for Wisconsin, was instrumental in influencing the passing the Proxmire Amendment. The Proxmire Amendment is also known as The Rogers-Proxmire Amendment of 1976, and The Vitamins and Minerals Amendments. This amendment became section 411 of the Federal Food, Drug, and Cosmetic Act.
O Positiv, Inc. is a healthcare company headquartered in Los Angeles. It specializes in dietary supplements aimed at easing the symptoms of premenstrual syndrome (PMS). The company is the owner of the FLO brand.









