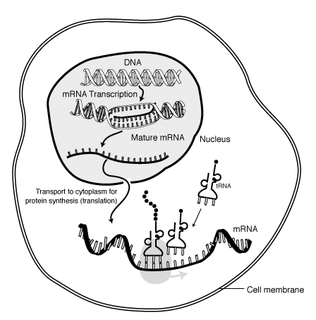
Protein synthesis is the process whereby biological cells generate new proteins; it is balanced by the loss of cellular proteins via degradation or export. Translation, the assembly of amino acids by ribosomes, is an essential part of the biosynthetic pathway, along with generation of messenger RNA (mRNA), aminoacylation of transfer RNA (tRNA), co-translational transport, and post-translational modification. Protein biosynthesis is strictly regulated at multiple steps. They are principally during transcription and translation.

An aminoacyl-tRNA synthetase, also called tRNA-ligase, is an enzyme that attaches the appropriate amino acid onto its tRNA. It does so by catalyzing the esterification of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. In humans, the 20 different types of aa-tRNA are made by the 20 different aminoacyl-tRNA synthetases, one for each amino acid of the genetic code.

Transfer-messenger RNA is a bacterial RNA molecule with dual tRNA-like and messenger RNA-like properties. The tmRNA forms a ribonucleoprotein complex (tmRNP) together with Small Protein B (SmpB), Elongation Factor Tu (EF-Tu), and ribosomal protein S1. In trans-translation, tmRNA and its associated proteins bind to bacterial ribosomes which have stalled in the middle of protein biosynthesis, for example when reaching the end of a messenger RNA which has lost its stop codon. The tmRNA is remarkably versatile: it recycles the stalled ribosome, adds a proteolysis-inducing tag to the unfinished polypeptide, and facilitates the degradation of the aberrant messenger RNA. In the majority of bacteria these functions are carried out by standard one-piece tmRNAs. In other bacterial species, a permuted ssrA gene produces a two-piece tmRNA in which two separate RNA chains are joined by base-pairing.
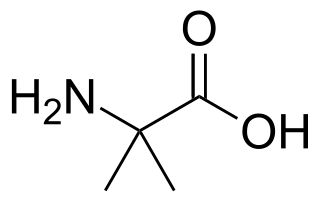
2-Aminoisobutyric acid, or α-aminoisobutyric acid (AIB) or α-methylalanine or 2-methylalanine, is the non-proteinogenic amino acid with the structural formula H2N-C(CH3)2-COOH. It is rare in nature only being found in some antibiotics of fungal origin, e.g. alamethicin and some lantibiotics.

The T-arm or T-loop is a specialized region on the tRNA molecule which acts as a special recognition site for the ribosome to form a tRNA-ribosome complex during protein biosynthesis or translation (biology).

The D arm is a feature in the tertiary structure of transfer RNA (tRNA). It is composed of the two D stems and the D loop. The D loop contains the base dihydrouridine, for which the arm is named. The D loop's main function is that of recognition. It is widely believed that it acts as a recognition site for aminoacyl-tRNA synthetase, an enzyme involved in the aminoacylation of the tRNA molecule. The D stem is also believed to have a recognition role although this is yet to be proved.

Glycine—tRNA ligase also known as glycyl-tRNA synthetase is an enzyme that in humans is encoded by the GARS gene.
In enzymology, a threonine-tRNA ligase is an enzyme that catalyzes the chemical reaction
Tyrosine—tRNA ligase, also known as tyrosyl-tRNA synthetase, is an enzyme that catalyzes the chemical reaction

Tryptophanyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the WARS gene.

Tyrosyl-tRNA synthetase, cytoplasmic, also known as Tyrosine-tRNA ligase, is an enzyme that in humans is encoded by the YARS gene.

Arginyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the RARS gene.

Probable leucyl-tRNA synthetase, mitochondrial is an enzyme that in humans is encoded by the LARS2 gene.

Tryptophanyl-tRNA synthetase, mitochondrial is an enzyme that in humans is encoded by the WARS2 gene.
Amino acid activation refers to the attachment of an amino acid to its Transfer RNA (tRNA).
Aminoacyl-tRNA synthetase, class II is a protein domain that catalyses the attachment of an amino acid to its cognate transfer RNA molecule in a highly specific two-step reaction. This protein differs widely in size and oligomeric state, and has a limited sequence homology.
The aminoacyl-tRNA synthetases catalyse the attachment of an amino acid to its cognate transfer RNA molecule in a highly specific two-step reaction. These proteins differ widely in size and oligomeric state, and have limited sequence homology. The 20 aminoacyl-tRNA synthetases are divided into two classes, I and II. Class I aminoacyl-tRNA synthetases contain a characteristic Rossman fold catalytic domain and are mostly monomeric. Class II aminoacyl-tRNA synthetases share an anti-parallel beta-sheet fold flanked by alpha-helices, and are mostly dimeric or multimeric, containing at least three conserved regions. However, tRNA binding involves an alpha-helical structure that is conserved between class I and class II synthetases. In reactions catalysed by the class I aminoacyl-tRNA synthetases, the aminoacyl group is coupled to the 2'-hydroxyl of the tRNA, while, in class II reactions, the 3'-hydroxyl site is preferred. The synthetases specific for arginine, cysteine, glutamic acid, glutamine, isoleucine, leucine, methionine, tyrosine, tryptophan and valine belong to class I synthetases; these synthetases are further divided into three subclasses, a, b and c, according to sequence homology. The synthetases specific for alanine, asparagine, aspartic acid, glycine, histidine, lysine, phenylalanine, proline, serine, and threonine belong to class-II synthetases.

Paul Reinhard Schimmel is an American biophysical chemist and translational medicine pioneer.

In molecular biology, Domain B5 is found in phenylalanine-tRNA synthetase beta subunits. This domain has been shown to bind DNA through a winged helix-turn-helix motif. Phenylalanine-tRNA synthetase may influence common cellular processes via DNA binding, in addition to its aminoacylation function.













