Aminobiphenyl may refer to:
| This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
Aminobiphenyl may refer to:
| This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
Cigarette smoke is an aerosol produced by the incomplete combustion of tobacco during the smoking of cigarettes. Temperatures in burning cigarettes range from about 400 ℃ between puffs to about 900 ℃ during a puff. During the burning of the cigarette tobacco, thousands of chemical substances are generated by combustion, distillation, pyrolysis and pyrosynthesis. Tobacco smoke is used as a fumigant and inhalant.

Hair coloring, or hair dyeing, is the practice of changing the hair color. The main reasons for this are cosmetic: to cover gray or white hair, to change to a color regarded as more fashionable or desirable, or to restore the original hair color after it has been discolored by hairdressing processes or sun bleaching.
Glucuronidation is often involved in drug metabolism of substances such as drugs, pollutants, bilirubin, androgens, estrogens, mineralocorticoids, glucocorticoids, fatty acid derivatives, retinoids, and bile acids. These linkages involve glycosidic bonds.
The Bischler–Napieralski reaction is an intramolecular electrophilic aromatic substitution reaction that allows for the cyclization of β-arylethylamides or β-arylethylcarbamates. It was first discovered in 1893 by August Bischler and Bernard Napieralski, in affiliation with Basle Chemical Works and the University of Zurich. The reaction is most notably used in the synthesis of dihydroisoquinolines, which can be subsequently oxidized to isoquinolines.
Pictet is the surname of several people:

Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidized impurities. Diphenylamine dissolves well in many common organic solvents, and is moderately soluble in water. It is used mainly for its antioxidant properties. Diphenylamine is widely used as an industrial antioxidant, dye mordant and reagent and is also employed in agriculture as a fungicide and antihelmintic.

Herbal cigarettes are cigarettes that usually do not contain any tobacco, instead being composed of a mixture of various herbs and/or other plant material. However, Chinese herbal cigarettes contain tobacco and nicotine with herbs added, unlike European and North American herbal cigarettes which have tobacco and nicotine omitted. Like herbal smokeless tobacco, they are often used as a substitute for standard tobacco products. Herbal cigarettes are considered a "non-smoking aid." European countries advertise herbal cigarettes as a cessation smoking aid. Herbal cigarettes are also used in acting scenes by performers who are non-smokers, or—as is becoming increasingly common—where anti-smoking legislation prohibits the use of tobacco in public spaces. Herbal cigarettes can carry carcinogens which can have health implications.
4-Aminobiphenyl (4-APB) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored. 4-Aminobiphenyl was commonly used in the past as a rubber antioxidant and an intermediate for dyes. Exposure to this aryl-amine can happen through contact with chemical dyes and from inhalation of cigarette smoke. Researches showed that 4-aminobiphenyl is responsible for bladder cancer in humans and dogs by damaging DNA. Due to its carcinogenic effects, commercial production of 4-aminobiphenyl ceased in the United States in the 1950s.

Sidestream smoke is smoke which goes into the air directly from a burning cigarette, cigar, or smoking pipe. Sidestream smoke is the main component of second-hand smoke (SHS), also known as Environmental Tobacco Smoke (ETS) or passive smoking. The chemical constituents of sidestream smoke are different from those of directly inhaled ("mainstream") smoke. Sidestream smoke has been classified as a Class A carcinogen by the U.S. Environmental Protection Agency.
In enzymology, an aromatic-hydroxylamine O-acetyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an arylamine N-acetyltransferase is an enzyme that catalyzes the chemical reaction

UDP glucuronosyltransferase 2 family, polypeptide B4, also known as UGT2B4, is an enzyme that in humans is encoded by the UGT2B4 gene.

Phenanthridine is a nitrogen heterocyclic compound that is the basis of DNA-binding fluorescent dyes through intercalation. Examples of such dyes are ethidium bromide and propidium iodide. Acridine is an isomer of phenanthridine.

XPhos is a phosphine ligand derived from biphenyl. Its palladium complexes exhibit high activity for Buchwald-Hartwig amination reactions involving aryl chlorides and aryl tosylates. Both palladium and copper complexes of the compound exhibit high activity for the coupling of aryl halides and aryl tosylates with various amides. It is also an efficient ligand for several commonly used C–C bond-forming cross-coupling reactions, including the Negishi, Suzuki, and the copper-free Sonogashira coupling reactions. It is especially efficient and general when employed as a (2-aminobiphenyl)-cyclometalated palladium mesylate precatalyst complex, XPhos-G3-Pd, which is commercially available and stable to bench storage. The ligand itself also has convenient handling characteristics as a crystalline, air-stable solid.
The molecular formula C12H11N may refer to:
Carbazole 1,9a-dioxygenase (EC 1.14.12.22, CARDO) is an enzyme with systematic name 9H-carbazole,NAD(P)H:oxygen oxidoreductase (2,3-hydroxylating). This enzyme catalyses the following chemical reaction
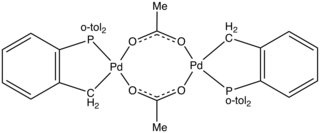
Herrmann's catalyst is an organopalladium compound that is a popular catalyst for the Heck reaction. It is a yellow air-stable solid that is soluble in organic solvents. Under conditions for catalysis, the acetate group is lost and the Pd-C bond undergoes protonolysis, giving rise to a source of "PdP(o-tol)3".

Lung cancer in Australia has killed more than 9,000 people and there are estimated to be over 12,500 new cases as of 2018. Lung cancer is the leading cause of cancer death in Australia and is responsible for one fifth of cancer diagnosis in the nation. It is differentiated into two different types: Non-small cell lung cancer and small cell-lung cancer. There are a range of diagnostic and treatment options available to treat both disease types. Smoking tobacco cigarettes is considered the leading risk factor of lung cancer in Australia, and Government-led public health schemes have aimed to reduce smoking and minimise its lung cancer risk. There has been relative success in these campaigns, and in treatment, as survival rates have improved from 9.2% to 17% as of 2014.

2-Aminobiphenyl (2-APB) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored even black. Palladacycles obtained from 2-aminobiphenyl are popular catalysts for cross-coupling.