
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula R−O−CH3.
A Lindlar catalyst is a heterogeneous catalyst consisting of palladium deposited on calcium carbonate or barium sulfate then poisoned with various forms of lead or sulfur. It is used for the hydrogenation of alkynes to alkenes. It is named after its inventor Herbert Lindlar, who discovered it in 1952.

In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.
The Ullmann condensation or Ullmann-type reaction is the copper-promoted conversion of aryl halides to aryl ethers, aryl thioethers, aryl nitriles, and aryl amines. These reactions are examples of cross-coupling reactions.

Palladium(II) acetate is a chemical compound of palladium described by the formula [Pd(O2CCH3)2]n, abbreviated [Pd(OAc)2]n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions.
The Hunsdiecker reaction is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide. It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting material and a halogen atom is introduced its place. A catalytic approach has been developed.
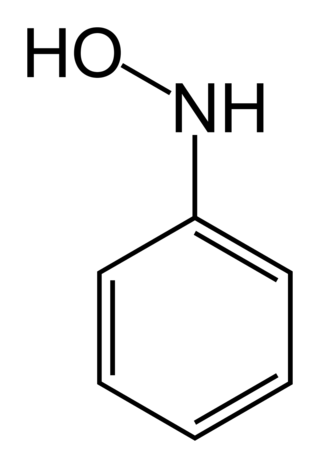
Phenylhydroxylamine is the organic compound with the formula C6H5NHOH. It is an intermediate in the redox-related pair C6H5NH2 and C6H5NO. Phenylhydroxylamine should not be confused with its isomer α-phenylhydroxylamine or O-phenylhydroxylamine.

Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.

Picryl chloride is an organic compound with the formula ClC6H2(NO2)3. It is a bright yellow solid that is highly explosive, as is typical for polynitro aromatics such as picric acid. Its detonation velocity is 7,200 m/s.
The Béchamp reduction is a chemical reaction that converts aromatic nitro compounds to their corresponding anilines using iron as the reductant:

Dichlorophenylphosphine is an organophosphorus compound with the formula C6H5PCl2. This colourless viscous liquid is commonly used in the synthesis of organophosphines.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.
Amide reduction is a reaction in organic synthesis where an amide is reduced to either an amine or an aldehyde functional group.

Dichloro[1,3-bis(diphenylphosphino)propane]nickel a coordination complex with the formula NiCl2(dppp); where dppp is the diphosphine 1,3-bis(diphenylphosphino)propane. It is used as a catalyst in organic synthesis. The compound is a bright orange-red crystalline powder.
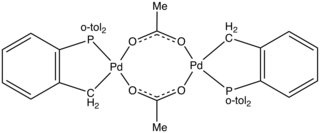
Herrmann's catalyst is an organopalladium compound that is a popular catalyst for the Heck reaction. It is a yellow air-stable solid that is soluble in organic solvents. Under conditions for catalysis, the acetate group is lost and the Pd-C bond undergoes protonolysis, giving rise to a source of "PdP(o-tol)3".
Palladacycle, as a class of metallacycles, refers to complexes containing at least one carbon-palladium bond. Palladacycles are invoked as intermediates in catalytic or palladium mediated reactions. They have been investigated as pre-catalysts for homogeneous catalysis and synthesis.

Tris(o-tolyl)phosphine is an organophosphorus compound with the formula P(C6H4CH3)3. It is a white, water-insoluble solid that is soluble in organic solvents. In solution it slowly converts to the phosphine oxide. As a phosphine ligand, it has a wide cone angle of 194°. Consequently, it tends to cyclometalate when treated with metal halides and metal acetates. Complexes of this ligand are common in homogeneous catalysis.
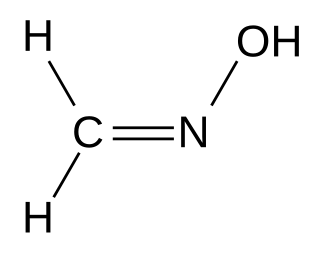
Formaldoxime is the organic compound with the formula H2C=N−OH. It is the oxime of formaldehyde. A colorless liquid, the pure compound tends to polymerize into a cyclic trimer. Aqueous solutions are stable as is the formaldoxime hydrochloride. It is a reagent in organic synthesis for the conversion of aryl diazonium salts to aryl aldehydes.
In cross-coupling reactions, the component reagents are called cross-coupling partners or simply coupling partners. These reagents can be further classified according to their nucleophilic vs electrophilic character:

n-Butylbenzene is the organic compound with the formula C6H5C4H9. Of two isomers of butylbenzene, n-butylbenzene consists of a phenyl group attached to the 1 position of a butyl group. It is a slightly greasy, colorless liquid.














