Related Research Articles

The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols.
The Perkin reaction is an organic reaction developed by English chemist William Henry Perkin that is used to make cinnamic acids. It gives an α,β-unsaturated aromatic acid by the aldol condensation of an aromatic aldehyde and an acid anhydride, in the presence of an alkali salt of the acid. The alkali salt acts as a base catalyst, and other bases can be used instead.

Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10.

Tetrahedron is a weekly peer-reviewed scientific journal covering the field of organic chemistry. According to the Journal Citation Reports, the journal has a 2014 impact factor of 2.641. Tetrahedron and Elsevier, its publisher, support an annual symposium. In 2010, complaints were raised over its high subscription cost.
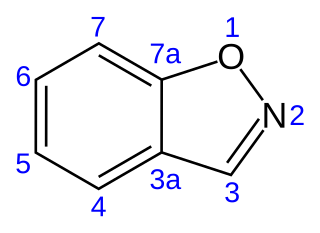
1,2-Benzisoxazole is an aromatic organic compound with a molecular formula C7H5NO containing a benzene-fused isoxazole ring structure. The compound itself has no common applications; however, functionalized benzisoxazoles and benzisoxazoyls have a variety of uses, including pharmaceutical drugs such as some antipsychotics (including risperidone, paliperidone, ocaperidone, and iloperidone) and the anticonvulsant zonisamide.

Trifluoromethanesulfonic anhydride, also known as triflic anhydride, is the chemical compound with the formula (CF3SO2)2O. It is the acid anhydride derived from triflic acid. This compound is a strong electrophile, useful for introducing the triflyl group, CF3SO2. Abbreviated Tf2O, triflic anhydride is the acid anhydride of the strong acid triflic acid, CF3SO2OH.

Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are produced by plants, and the only known exception is the antihelminthic and antimicrobial stilbenoid, 2-isoprpyl-5-[(E)-2-phenylvinyl]benzene-1,3-diol, biosynthesized by the Gram-negative bacterium Photorhabdus luminescens.
The Étard reaction is a chemical reaction that involves the direct oxidation of an aromatic or heterocyclic bound methyl group to an aldehyde using chromyl chloride. For example, toluene can be oxidized to benzaldehyde.
Daniel Schaeffer Kemp was an American organic chemist, an emeritus professor of chemistry at the Massachusetts Institute of Technology. Kemp's work was focused on the synthesis and conformational analysis of peptides. He developed several chemical ligation strategies and methods for templating the formation of helices and sheets. The eponymous Kemp's triacid and the Kemp elimination reaction are among his developments. He was the author of an organic chemistry textbook.

Ampelopsis glandulosa var. brevipedunculata, with common names creeper, porcelain berry, Amur peppervine, and wild grape, is an ornamental plant, native to temperate areas of Asia. It is generally similar to, and potentially confused with, grape species and other Ampelopsis species.
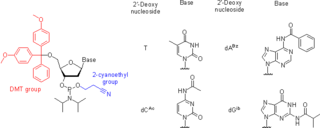
Nucleoside phosphoramidites are derivatives of natural or synthetic nucleosides. They are used to synthesize oligonucleotides, relatively short fragments of nucleic acid and their analogs. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers. To avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected. As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids. To be incorporated in the middle of an oligonucleotide chain using phosphoramidite strategy, the nucleoside analog must possess two hydroxy groups or, less often, a hydroxy group and another nucleophilic group (amino or mercapto). Examples include, but are not limited to, alternative nucleotides, LNA, morpholino, nucleosides modified at the 2'-position (OMe, protected NH2, F), nucleosides containing non-canonical bases (hypoxanthine and xanthine contained in natural nucleosides inosine and xanthosine, respectively, tricyclic bases such as G-clamp, etc.) or bases derivatized with a fluorescent group or a linker arm.

Cyclobutanetetrone, also called tetraoxocyclobutane, is an organic compound with formula C4O4 or (CO)4, the fourfold ketone of cyclobutane. It would be an oxide of carbon, indeed a tetramer of carbon monoxide.

Eupatolitin is a chemical compound. It is an O-methylated flavonol, a type of flavonoid. Eupatolitin can be found in Brickellia veronicaefolia and in Ipomopsis aggregata.
The Barton decarboxylation is a radical reaction in which a carboxylic acid is converted to a thiohydroxamate ester. The product is then heated in the presence of a radical initiator and a suitable hydrogen donor to afford the decarboxylated product. This is an example of a reductive decarboxylation. Using this reaction it is possible to remove carboxylic acid moieties from alkyl groups and replace them with other functional groups. This reaction is named after its developer, the British chemist and Nobel laureate Sir Derek Barton (1918–1998).

Ampelopsin B is a stilbenoid dimer found in Ampelopsis glandulosa var. hancei.
Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound. Trifluoromethylated compounds are of some importance in pharmaceutical industry and agrochemicals. Several notable pharmaceutical compounds have a trifluoromethyl group incorporated: fluoxetine, mefloquine, Leflunomide, nulitamide, dutasteride, bicalutamide, aprepitant, celecoxib, fipronil, fluazinam, penthiopyrad, picoxystrobin, fluridone, norflurazon, sorafenib and triflurazin. A relevant agrochemical is trifluralin. The development of synthetic methods for adding trifluoromethyl groups to chemical compounds is actively pursued in academic research.

Visnagin is an organic chemical compound with the molecular formula C13H10O4 It is a furanochromone, a compound derivative of chromone (1,4-benzopyrone) and furan.

Aknadinine is an opioid alkaloid isolated from members of the genus Stephania. Structurally it is a member of the hasubanan family of alkaloids and features an isoquinoline substructure.
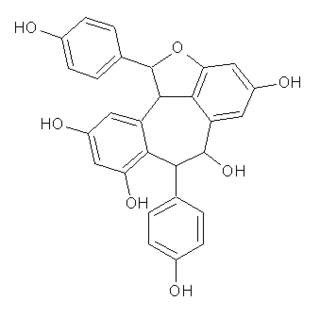
Ampelopsin A is a resveratrol dimer found in Ampelopsis glandulosa var. hancei.
Penicillium brefeldianum is an anamorph fungus species of the genus of Penicillium which produces Brefeldin A a fungal metabolite.
References
- 1 2 3 Oshima, Y. (1990). "Ampelopsins A, B and C, new oligostilbenes of ? ? VAR. ?". Tetrahedron. 46 (15): 5121–5126. doi:10.1016/S0040-4020(01)87819-4.
- 1 2 3 4 Takaya, Y. (2002). "Chemical determination of the absolute structures of resveratrol dimers, ampelopsins A, B, D and F". Tetrahedron. 58 (36): 7259–7265. doi:10.1016/S0040-4020(02)00785-8.
- ↑ Cai, T.; Cai, Y. (2011). "Cis-Ampelopsin E, a stilbene isolated from the seeds of Paeonia suffruticosa, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via blockade of nuclear factor-kappa B signaling pathway". Biological & Pharmaceutical Bulletin. 34 (9): 1501–1507. doi: 10.1248/bpb.34.1501 . PMID 21881241.
- ↑ Ampelopsin F on PubChem