Related Research Articles

The atom is the basic particle of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element.

In condensed matter physics, a Bose–Einstein condensate (BEC) is a state of matter that is typically formed when a gas of bosons at very low densities is cooled to temperatures very close to absolute zero. Under such conditions, a large fraction of bosons occupy the lowest quantum state, at which microscopic quantum mechanical phenomena, particularly wavefunction interference, become apparent macroscopically. More generally, condensation refers to the appearance of macroscopic occupation of one or several states: for example, in BCS theory, a superconductor is a condensate of Cooper pairs. As such, condensation can be associated with phase transition, and the macroscopic occupation of the state is the order parameter.

In atomic physics, the Bohr model or Rutherford–Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons. It is analogous to the structure of the Solar System, but with attraction provided by electrostatic force rather than gravity, and with the electron energies quantized.

The electron is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy the same quantum state, per the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy.

A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe.
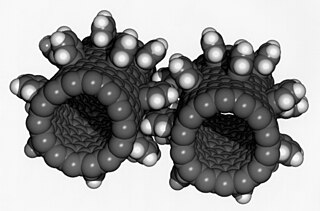
Nanotechnology was defined by the National Nanotechnology Initiative as the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). At this scale, commonly known as the nanoscale, surface area and quantum mechanical effects become important in describing properties of matter. The definition of nanotechnology is inclusive of all types of research and technologies that deal with these special properties. It is therefore common to see the plural form "nanotechnologies" as well as "nanoscale technologies" to refer to the broad range of research and applications whose common trait is size. An earlier description of nanotechnology referred to the particular technological goal of precisely manipulating atoms and molecules for fabrication of macroscale products, also now referred to as molecular nanotechnology.
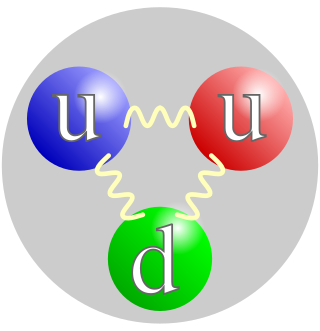
A proton is a stable subatomic particle, symbol
p
, H+, or 1H+ with a positive electric charge of +1 e (elementary charge). Its mass is slightly less than the mass of a neutron and 1,836 times the mass of an electron (the proton-to-electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei).

The Geiger–Marsden experiments were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. They deduced this after measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The experiments were performed between 1908 and 1913 by Hans Geiger and Ernest Marsden under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester.
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.

In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Quantum numbers are closely related to eigenvalues of observables. When the corresponding observable commutes with the Hamiltonian, the quantum number is said to be "good", and acts as a constant of motion in the quantum dynamics.
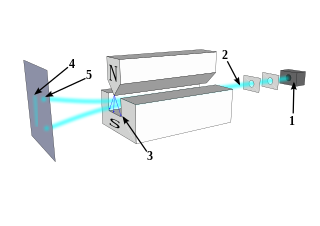
In quantum physics, the Stern–Gerlach experiment demonstrated that the spatial orientation of angular momentum is quantized. Thus an atomic-scale system was shown to have intrinsically quantum properties. In the original experiment, silver atoms were sent through a spatially-varying magnetic field, which deflected them before they struck a detector screen, such as a glass slide. Particles with non-zero magnetic moment were deflected, owing to the magnetic field gradient, from a straight path. The screen revealed discrete points of accumulation, rather than a continuous distribution, owing to their quantized spin. Historically, this experiment was decisive in convincing physicists of the reality of angular-momentum quantization in all atomic-scale systems.
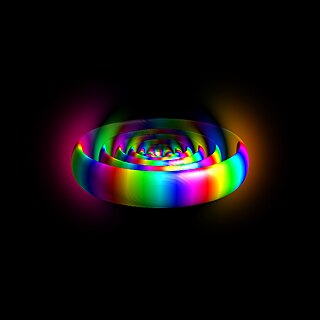
A Rydberg atom is an excited atom with one or more electrons that have a very high principal quantum number, n. The higher the value of n, the farther the electron is from the nucleus, on average. Rydberg atoms have a number of peculiar properties including an exaggerated response to electric and magnetic fields, long decay periods and electron wavefunctions that approximate, under some conditions, classical orbits of electrons about the nuclei. The core electrons shield the outer electron from the electric field of the nucleus such that, from a distance, the electric potential looks identical to that experienced by the electron in a hydrogen atom.
Although there are nine known isotopes of helium (2He), only helium-3 and helium-4 are stable. All radioisotopes are short-lived, the longest-lived being 6
He
with a half-life of 806.92(24) milliseconds. The least stable is 10
He
, with a half-life of 260(40) yoctoseconds, although it is possible that 2
He
may have an even shorter half-life.
Atom optics "refers to techniques to manipulate the trajectories and exploit the wave properties of neutral atoms". Typical experiments employ beams of cold, slowly moving neutral atoms, as a special case of a particle beam. Like an optical beam, the atomic beam may exhibit diffraction and interference, and can be focused with a Fresnel zone plate or a concave atomic mirror.
The Planck constant, or Planck's constant, denoted by , is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a matter wave equals the Planck constant divided by the associated particle momentum.

A trojan wave packet is a wave packet that is nonstationary and nonspreading. It is part of an artificially created system that consists of a nucleus and one or more electron wave packets, and that is highly excited under a continuous electromagnetic field. Its discovery as one of significant contributions to the Quantum Theory was awarded the 2022 Wigner Medal for Iwo Bialynicki-Birula

An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions between such states they interact with a very specific frequency of electromagnetic radiation. This phenomenon serves as the basis for the International System of Units' (SI) definition of a second:
The second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency, , the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be 9192631770 when expressed in the unit Hz, which is equal to s−1.
Quantum microscopy allows microscopic properties of matter and quantum particles to be measured and imaged. Various types of microscopy use quantum principles. The first microscope to do so was the scanning tunneling microscope, which paved the way for development of the photoionization microscope and the quantum entanglement microscope.

Photek Limited is a specialist manufacturer and global supplier of vacuum-based tubes and camera systems for photon detection. Photek manufacture image intensifiers, solar-blind detectors, photomultipliers, streak tubes and a range of associated electronics and camera systems. The company was founded in 1991 by Jon Howorth, Ralph Powell, Martin Ingle, Geoff Holt and Mehmet Madakbas.
Benjamin Leonard Lev is an American physicist and Professor of Physics and Applied Physics at Stanford University. He studies quantum many-body physics, both in and out of equilibrium, by combining the tools of ultracold atomic physics, quantum optics, and condensed matter physics.
References
- ↑ "Snapshots of atoms make it into physics textbooks" . Retrieved 2017-08-26.
- ↑ "Smile, hydrogen atom, you're on quantum camera". New Scientist. Retrieved 2017-08-26.
- ↑ Stodolna, A. S.; Rouzée, A.; Lépine, F.; Cohen, S.; Robicheaux, F.; Gijsbertsen, A.; Jungmann, J. H.; Bordas, C.; Vrakking, M. J. J. (2013-05-20). "Hydrogen Atoms under Magnification: Direct Observation of the Nodal Structure of Stark States" (PDF). Physical Review Letters. 110 (21): 213001. Bibcode:2013PhRvL.110u3001S. doi: 10.1103/PhysRevLett.110.213001 . PMID 23745864.
- ↑ "'Quantum microscope' peers into the hydrogen atom - physicsworld.com". physicsworld.com. 23 May 2013. Retrieved 2017-08-26.
- ↑ "Cosmic neutrinos named Physics World 2013 Breakthrough of the Year - physicsworld.com". physicsworld.com. 13 December 2013. Retrieved 2017-08-26.