
The optical microscope, also referred to as a light microscope, is a type of microscope that commonly uses visible light and a system of lenses to generate magnified images of small objects. Optical microscopes are the oldest design of microscope and were possibly invented in their present compound form in the 17th century. Basic optical microscopes can be very simple, although many complex designs aim to improve resolution and sample contrast.

A karyotype is the general appearance of the complete set of chromosomes in the cells of a species or in an individual organism, mainly including their sizes, numbers, and shapes. Karyotyping is the process by which a karyotype is discerned by determining the chromosome complement of an individual, including the number of chromosomes and any abnormalities.

Cytogenetics is essentially a branch of genetics, but is also a part of cell biology/cytology, that is concerned with how the chromosomes relate to cell behaviour, particularly to their behaviour during mitosis and meiosis. Techniques used include karyotyping, analysis of G-banded chromosomes, other cytogenetic banding techniques, as well as molecular cytogenetics such as fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH).

Flow cytometry (FC) is a technique used to detect and measure the physical and chemical characteristics of a population of cells or particles.
Comparative genomic hybridization (CGH) is a molecular cytogenetic method for analysing copy number variations (CNVs) relative to ploidy level in the DNA of a test sample compared to a reference sample, without the need for culturing cells. The aim of this technique is to quickly and efficiently compare two genomic DNA samples arising from two sources, which are most often closely related, because it is suspected that they contain differences in terms of either gains or losses of either whole chromosomes or subchromosomal regions. This technique was originally developed for the evaluation of the differences between the chromosomal complements of solid tumor and normal tissue, and has an improved resolution of 5–10 megabases compared to the more traditional cytogenetic analysis techniques of giemsa banding and fluorescence in situ hybridization (FISH) which are limited by the resolution of the microscope utilized.

Fluorescence in situ hybridization (FISH) is a molecular cytogenetic technique that uses fluorescent probes that bind to only particular parts of a nucleic acid sequence with a high degree of sequence complementarity. It was developed by biomedical researchers in the early 1980s to detect and localize the presence or absence of specific DNA sequences on chromosomes. Fluorescence microscopy can be used to find out where the fluorescent probe is bound to the chromosomes. FISH is often used for finding specific features in DNA for use in genetic counseling, medicine, and species identification. FISH can also be used to detect and localize specific RNA targets in cells, circulating tumor cells, and tissue samples. In this context, it can help define the spatial-temporal patterns of gene expression within cells and tissues.

Multispectral imaging captures image data within specific wavelength ranges across the electromagnetic spectrum. The wavelengths may be separated by filters or detected with the use of instruments that are sensitive to particular wavelengths, including light from frequencies beyond the visible light range. It can allow extraction of additional information the human eye fails to capture with its visible receptors for red, green and blue. It was originally developed for military target identification and reconnaissance. Early space-based imaging platforms incorporated multispectral imaging technology to map details of the Earth related to coastal boundaries, vegetation, and landforms. Multispectral imaging has also found use in document and painting analysis.
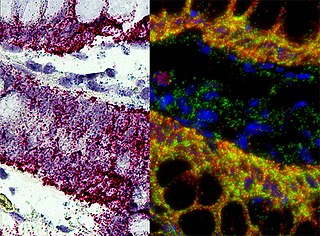
In situ hybridization (ISH) is a type of hybridization that uses a labeled complementary DNA, RNA or modified nucleic acid strand to localize a specific DNA or RNA sequence in a portion or section of tissue or if the tissue is small enough, in the entire tissue, in cells, and in circulating tumor cells (CTCs). This is distinct from immunohistochemistry, which usually localizes proteins in tissue sections.

Hyperspectral imaging collects and processes information from across the electromagnetic spectrum. The goal of hyperspectral imaging is to obtain the spectrum for each pixel in the image of a scene, with the purpose of finding objects, identifying materials, or detecting processes. There are three general types of spectral imagers. There are push broom scanners and the related whisk broom scanners, which read images over time, band sequential scanners, which acquire images of an area at different wavelengths, and snapshot hyperspectral imagers, which uses a staring array to generate an image in an instant.

Molecular cytogenetics combines two disciplines, molecular biology and cytogenetics, and involves the analysis of chromosome structure to help distinguish normal and cancer-causing cells. Human cytogenetics began in 1956 when it was discovered that normal human cells contain 46 chromosomes. However, the first microscopic observations of chromosomes were reported by Arnold, Flemming, and Hansemann in the late 1800s. Their work was ignored for decades until the actual chromosome number in humans was discovered as 46. In 1879, Arnold examined sarcoma and carcinoma cells having very large nuclei. Today, the study of molecular cytogenetics can be useful in diagnosing and treating various malignancies such as hematological malignancies, brain tumors, and other precursors of cancer. The field is overall focused on studying the evolution of chromosomes, more specifically the number, structure, function, and origin of chromosome abnormalities. It includes a series of techniques referred to as fluorescence in situ hybridization, or FISH, in which DNA probes are labeled with different colored fluorescent tags to visualize one or more specific regions of the genome. Introduced in the 1980s, FISH uses probes with complementary base sequences to locate the presence or absence of the specific DNA regions. FISH can either be performed as a direct approach to metaphase chromosomes or interphase nuclei. Alternatively, an indirect approach can be taken in which the entire genome can be assessed for copy number changes using virtual karyotyping. Virtual karyotypes are generated from arrays made of thousands to millions of probes, and computational tools are used to recreate the genome in silico.
Chemical imaging is the analytical capability to create a visual image of components distribution from simultaneous measurement of spectra and spatial, time information. Hyperspectral imaging measures contiguous spectral bands, as opposed to multispectral imaging which measures spaced spectral bands.

Airborne Real-time Cueing Hyperspectral Enhanced Reconnaissance, also known by the acronym ARCHER, is an aerial imaging system that produces ground images far more detailed than plain sight or ordinary aerial photography can. It is the most sophisticated unclassified hyperspectral imaging system available, according to U.S. Government officials. ARCHER can automatically scan detailed imaging for a given signature of the object being sought, for abnormalities in the surrounding area, or for changes from previous recorded spectral signatures.
Electro-optical MASINT is a subdiscipline of Measurement and Signature Intelligence, (MASINT) and refers to intelligence gathering activities which bring together disparate elements that do not fit within the definitions of Signals Intelligence (SIGINT), Imagery Intelligence (IMINT), or Human Intelligence (HUMINT).

Digital pathology is a sub-field of pathology that focuses on managing and analyzing information generated from digitized specimen slides. It utilizes computer-based technology and virtual microscopy to view, manage, share, and analyze digital slides on computer monitors. This field has applications in diagnostic medicine and aims to achieve more efficient and cost-effective diagnoses, prognoses, and disease predictions through advancements in machine learning and artificial intelligence in healthcare.

Automated tissue image analysis or histopathology image analysis (HIMA) is a process by which computer-controlled automatic test equipment is used to evaluate tissue samples, using computations to derive quantitative measurements from an image to avoid subjective errors.
In genetics, virtual karyotype is the digital information reflecting a karyotype, resulting from the analysis of short sequences of DNA from specific loci all over the genome, which are isolated and enumerated. It detects genomic copy number variations at a higher resolution for level than conventional karyotyping or chromosome-based comparative genomic hybridization (CGH). The main methods used for creating virtual karyotypes are array-comparative genomic hybridization and SNP arrays.
Chromogenic in situ hybridization (CISH) is a cytogenetic technique that combines the chromogenic signal detection method of immunohistochemistry (IHC) techniques with in situ hybridization. It was developed around the year 2000 as an alternative to fluorescence in situ hybridization (FISH) for detection of HER-2/neu oncogene amplification. CISH is similar to FISH in that they are both in situ hybridization techniques used to detect the presence or absence of specific regions of DNA. However, CISH is much more practical in diagnostic laboratories because it uses bright-field microscopes rather than the more expensive and complicated fluorescence microscopes used in FISH.
CytoViva, Inc. is a scientific imaging and instrumentation company that develops and markets optical microscopy and hyperspectral imaging technology for nanomaterials, pathogen and general biology applications.
Spatio-spectral scanning is one of four techniques for hyperspectral imaging, the other three being spatial scanning, spectral scanning and non-scanning, or snapshot hyperspectral imaging.
Tissue image cytometry or tissue cytometry is a method of digital histopathology and combines classical digital pathology and computational pathology into one integrated approach with solutions for all kinds of diseases, tissue and cell types as well as molecular markers and corresponding staining methods to visualize these markers. Tissue cytometry uses virtual slides as they can be generated by multiple, commercially available slide scanners, as well as dedicated image analysis software – preferentially including machine and deep learning algorithms. Tissue cytometry enables cellular analysis within thick tissues, retaining morphological and contextual information, including spatial information on defined cellular subpopulations.












