
A blood type is a classification of blood, based on the presence and absence of antibodies and inherited antigenic substances on the surface of red blood cells (RBCs). These antigens may be proteins, carbohydrates, glycoproteins, or glycolipids, depending on the blood group system. Some of these antigens are also present on the surface of other types of cells of various tissues. Several of these red blood cell surface antigens can stem from one allele and collectively form a blood group system.

Blood transfusion is the process of transferring blood products into a person's circulation intravenously. Transfusions are used for various medical conditions to replace lost components of the blood. Early transfusions used whole blood, but modern medical practice commonly uses only components of the blood, such as red blood cells, white blood cells, plasma, platelets, and other clotting factors.

A blood bank is a center where blood gathered as a result of blood donation is stored and preserved for later use in blood transfusion. The term "blood bank" typically refers to a department of a hospital usually within a clinical pathology laboratory where the storage of blood product occurs and where pre-transfusion and blood compatibility testing is performed. However, it sometimes refers to a collection center, and some hospitals also perform collection. Blood banking includes tasks related to blood collection, processing, testing, separation, and storage.
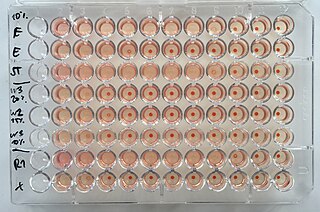
The hemagglutination assay or haemagglutination assay (HA) and the hemagglutination inhibition assay were developed in 1941–42 by American virologist George Hirst as methods for quantifying the relative concentration of viruses, bacteria, or antibodies.
Leukocyte adhesion deficiency (LAD) is a rare autosomal recessive disorder characterized by immunodeficiency resulting in recurrent infections. LAD is currently divided into three subtypes: LAD1, LAD2, and the recently described LAD3, also known as LAD-1/variant. In LAD3, the immune defects are supplemented by a Glanzmann thrombasthenia-like bleeding tendency.
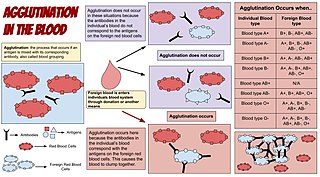
Agglutination is the clumping of particles. The word agglutination comes from the Latin agglutinare.
Autoimmune hemolytic anemia (AIHA) occurs when antibodies directed against the person's own red blood cells (RBCs) cause them to burst (lyse), leading to an insufficient number of oxygen-carrying red blood cells in the circulation. The lifetime of the RBCs is reduced from the normal 100–120 days to just a few days in serious cases. The intracellular components of the RBCs are released into the circulating blood and into tissues, leading to some of the characteristic symptoms of this condition. The antibodies are usually directed against high-incidence antigens, therefore they also commonly act on allogenic RBCs. AIHA is a relatively rare condition, with an incidence of 5–10 cases per 1 million persons per year in the warm-antibody type and 0.45 to 1.9 cases per 1 million persons per year in the cold antibody type. Autoimmune hemolysis might be a precursor of later onset systemic lupus erythematosus.

Francis Peyton Rous was an American pathologist at the Rockefeller University known for his works in oncoviruses, blood transfusion and physiology of digestion. A medical graduate from the Johns Hopkins University, he was discouraged from becoming a practicing physician due to severe tuberculosis. After three years of working as an instructor of pathology at the University of Michigan, he became dedicated researcher at the Rockefeller Institute for Medical Research for the rest of his career.
CLEC4C is a membrane protein of plasmacytoid dendritic cells used as a marker for this kind of cells and denoted as CD303 in the nomenclature of the Cluster of differentiation.

Integrin, alpha E (ITGAE) also known as CD103 is an integrin protein that in human is encoded by the ITGAE gene. CD103 binds integrin beta 7 to form the complete heterodimeric integrin molecule αEβ7, which has no distinct name. The αEβ7 complex is often referred to as "CD103" though this strictly refers only to the αE chain. Note that the β7 subunit can bind with other integrin α chains, such as α4 (CD49d).

Leukotriene B4 receptor 1 is a protein that in humans is encoded by the LTB4R gene.

Killer cell immunoglobulin-like receptor 2DL4 is a protein that in humans is encoded by the KIR2DL4 gene.
Immunoglobulin lambda-like polypeptide 1 is a protein that in humans is encoded by the IGLL1 gene. IGLL1 has also recently been designated CD179B.

Natural cytotoxicity triggering receptor 1 is a protein that in humans is encoded by the NCR1 gene. NCR1 has also been designated as CD335 (cluster of differentiation, NKP46, NKp46, NK-p46, and LY94.

T-cell receptor-associated transmembrane adapter 1 is a protein that in humans is encoded by the TRAT1 gene.
Transitional B cells are B cells at an intermediate stage in their development between bone marrow immature cells and mature B cells in the spleen. Primary B cell development takes place in the bone marrow, where immature B cells must generate a functional B cell receptor (BCR) and overcome negative selection induced by reactivity with autoantigens. Transitional cells can be found in the bone marrow, peripheral blood, and spleen, and only a fraction of the immature B cells that survive after the transitional stage become mature B cells in secondary lymphoid organs such as the spleen.

Women of Mayo Clinic: The Founding Generation is a 2016 non-fiction book by Virginia M. Wright-Peterson, chronicling the individual contributions of professional women who helped establish and develop the Mayo Clinic in Rochester, Minnesota. Covering a period of 60 years, the Sisters of Saint Francis of Rochester, Minnesota worked in conjunction with the Mayo family to open a hospital that would accept patients of all faiths. Beginning with a 27-bed facility, the women physicians and other medical professionals would eventually serve in theaters of war, and create an environment that evolved according to patient needs. Wright-Peterson is a faculty member of the University of Minnesota Rochester, and a former Mayo Clinic administrator.

Blood compatibility testing is conducted in a medical laboratory to identify potential incompatibilities between blood group systems in blood transfusion. It is also used to diagnose and prevent some complications of pregnancy that can occur when the baby has a different blood group from the mother. Blood compatibility testing includes blood typing, which detects the antigens on red blood cells that determine a person's blood type; testing for unexpected antibodies against blood group antigens ; and, in the case of blood transfusions, mixing the recipient's plasma with the donor's red blood cells to detect incompatibilities (crossmatching). Routine blood typing involves determining the ABO and RhD type, and involves both identification of ABO antigens on red blood cells and identification of ABO antibodies in the plasma. Other blood group antigens may be tested for in specific clinical situations.

Ludvig Hektoen was an American pathologist known for his work in the fields of pathology, microbiology and immunology. Hektoen was appointed to the National Academy of Sciences in 1918, and served as president of many professional societies, including the American Association of Immunologists in 1927 and the American Society for Microbiology in 1929. He was the founding editor of the Archives of Pathology and Laboratory Medicine in 1926 and edited several other medical journals. He was knighted to the Order of St. Olav in 1929, and in 1933, he became professor emeritus of pathology at the University of Chicago. The Hektoen Institute for Medical Research—formerly the John McCormick Institute of Infectious Diseases—now bears his name.

The monocyte monolayer assay (MMA) is used to determine the clinical significance of alloantibodies produced by blood transfusion recipients. The assay is used to assess the potential for intravascular hemolysis when incompatible cellular blood products are transfused to the anemic patient. When donor cells possess substances that are not produced by the recipient, the recipient's immune system produces antibodies against the substance; these are called alloantibodies. Specific white blood cells, called monocytes, are tasked with ingesting foreign material and become activated during certain inflammatory events. These activated monocytes come in contact with antibody-sensitized red blood cells (RBC) and may or may not exhibit phagocytosis (ingestion) and destroy the donor red blood cells. If monocytes destroy the RBC, the antibody attached to those RBC is considered clinically significant.
















