Related Research Articles
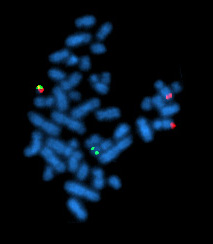
The Philadelphia chromosome or Philadelphia translocation (Ph) is a specific genetic abnormality in chromosome 22 of leukemia cancer cells. This chromosome is defective and unusually short because of reciprocal translocation, t(9;22)(q34;q11), of genetic material between chromosome 9 and chromosome 22, and contains a fusion gene called BCR-ABL1. This gene is the ABL1 gene of chromosome 9 juxtaposed onto the breakpoint cluster region BCR gene of chromosome 22, coding for a hybrid protein: a tyrosine kinase signaling protein that is "always on", causing the cell to divide uncontrollably by interrupting the stability of the genome and impairing various signaling pathways governing the cell cycle.

Chronic myelogenous leukemia (CML), also known as chronic myeloid leukemia, is a cancer of the white blood cells. It is a form of leukemia characterized by the increased and unregulated growth of myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which a proliferation of mature granulocytes and their precursors is found; characteristic increase in basophils is clinically relevant. It is a type of myeloproliferative neoplasm associated with a characteristic chromosomal translocation called the Philadelphia chromosome.

Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral targeted therapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple tyrosine kinases such as CSF1R, ABL, c-KIT, FLT3, and PDGFR-β. Specifically, it is used for chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) that are Philadelphia chromosome–positive (Ph+), certain types of gastrointestinal stromal tumors (GIST), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), systemic mastocytosis, and myelodysplastic syndrome.
K562 cells were the first human immortalised myelogenous leukemia cell line to be established. K562 cells are of the erythroleukemia type, and the cell line is derived from a 53-year-old female chronic myelogenous leukemia patient in blast crisis. The cells are non-adherent and rounded, are positive for the bcr:abl fusion gene, and bear some proteomic resemblance to both undifferentiated granulocytes and erythrocytes.

Nilotinib, sold under the brand name Tasigna marketed worldwide by Novartis, is a medication used to treat chronic myelogenous leukemia (CML) which has the Philadelphia chromosome. It may be used both in initial cases of chronic phase CML as well as in accelerated and chronic phase CML that has not responded to imatinib. It is taken by mouth.

Dasatinib, sold under the brand name Sprycel among others, is a targeted therapy medication used to treat certain cases of chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL). Specifically it is used to treat cases that are Philadelphia chromosome-positive (Ph+). It is taken by mouth.

Chemokine ligand 9 (CCL9) is a small cytokine belonging to the CC chemokine family. It is also called macrophage inflammatory protein-1 gamma (MIP-1γ), macrophage inflammatory protein-related protein-2 (MRP-2) and CCF18, that has been described in rodents. CCL9 has also been previously designated CCL10, although this name is no longer in use. It is secreted by follicle-associated epithelium (FAE) such as that found around Peyer's patches, and attracts dendritic cells that possess the cell surface molecule CD11b and the chemokine receptor CCR1. CCL9 can activate osteoclasts through its receptor CCR1 suggesting an important role for CCL9 in bone resorption. CCL9 is constitutively expressed in macrophages and myeloid cells. The gene for CCL9 is located on chromosome 11 in mice.

Acute myeloblastic leukemia with maturation (M2) is a subtype of acute myeloid leukemia (AML).
Accelerated phase chronic myelogenous leukemia is a phase of chronic myelogenous leukemia in which the disease is progressing. In this phase, 10 to 19% of the cells in the blood and bone marrow are blast cells. In the accelerated phase, these leukemia cells grow quickly.
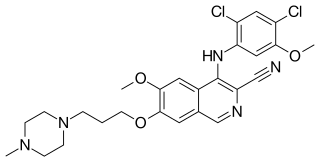
Bosutinib, sold under the brand name Bosulif, is a small molecule BCR-ABL and src tyrosine kinase inhibitor used for the treatment of chronic myelogenous leukemia.

Omacetaxine mepesuccinate, formerly named as homoharringtonine or HHT, is a pharmaceutical drug substance that is indicated for treatment of chronic myeloid leukemia (CML).

Brian J. Druker is a physician-scientist at Oregon Health & Science University (OHSU), in Portland, Oregon. He is the director of OHSU's Knight Cancer Institute, JELD-WEN Chair of Leukemia Research, Associate Dean for Oncology in the OHSU School of Medicine, and professor of medicine.

Nicholas B. Lydon FRS is a British scientist and entrepreneur. In 2009, he was awarded the Lasker Clinical Award and in 2012 the Japan Prize for the development of Gleevec, also known as Imatinib, a selective BCR-ABL inhibitor for the treatment of chronic myeloid leukaemia (CML), which converted a fatal cancer into a manageable chronic condition.

Carlo Gambacorti-Passerini is an Italian oncologist and hematologist known for his contributions to cancer research.
Bcr-Abl tyrosine-kinase inhibitors (TKI) are the first-line therapy for most patients with chronic myelogenous leukemia (CML). More than 90% of CML cases are caused by a chromosomal abnormality that results in the formation of a so-called Philadelphia chromosome. This abnormality was discovered by Peter Nowell in 1960 and is a consequence of fusion between the Abelson (Abl) tyrosine kinase gene at chromosome 9 and the break point cluster (Bcr) gene at chromosome 22, resulting in a chimeric oncogene (Bcr-Abl) and a constitutively active Bcr-Abl tyrosine kinase that has been implicated in the pathogenesis of CML. Compounds have been developed to selectively inhibit the tyrosine kinase.

SET binding protein 1 is a protein that in humans is encoded by the SETBP1 gene.

Ponatinib, sold under the brand name Iclusig, is a medication developed by ARIAD Pharmaceuticals for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). It is a multi-targeted tyrosine-kinase inhibitor. Some forms of CML, those that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.

Radotinib (INN; trade name Supect), and sometimes referred to by its investigational name IY5511, is a drug for the treatment of different types of cancer, most notably Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) with resistance or intolerance of other Bcr-Abl tyrosine-kinase inhibitors, such as patients resistant or intolerant to imatinib.
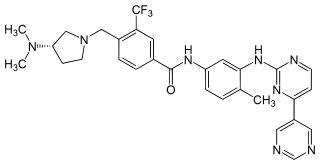
Bafetinib (NS-187) is an experimental cancer drug developed by Nippon Shinyaku and licensed to CytRx. It is an inhibitor of Lyn and Bcr-Abl. It reached phase II clinical trials in 2010.
Clonal hypereosinophilia, also termed primary hypereosinophilia or clonal eosinophilia, is a grouping of hematological disorders all of which are characterized by the development and growth of a pre-malignant or malignant population of eosinophils, a type of white blood cell that occupies the bone marrow, blood, and other tissues. This population consists of a clone of eosinophils, i.e. a group of genetically identical eosinophils derived from a sufficiently mutated ancestor cell.
References
- ↑ Vardiman, James W.; Thiele, Jüergen; Arber, Daniel A.; Brunning, Richard D.; Borowitz, Michael J.; Porwit, Anna; Harris, Nancy Lee; Le Beau, Michelle M.; Hellström-Lindberg, Eva; Tefferi, Ayalew; Bloomfield, Clara D. (July 30, 2009). "The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes". Blood. American Society of Hematology. 114 (5): 937–951. doi: 10.1182/blood-2009-03-209262 . ISSN 0006-4971.
- ↑ Nowell, Peter C.; Hungerford, David A. (1960). "A minute chromosome in human chronic granulocytic leukemia" (PDF). Science. 142: 1497. Retrieved 24 October 2023.
- ↑ Kurzrock, Razelle; Bueso-Ramos, Carlos E.; Kantarjian, Hagop; Freireich, Emil; Tucker, Susan L.; Siciliano, Michael; Pilat, Susan; Talpaz, Moshe (June 1, 2001). "BCR Rearrangement–Negative Chronic Myelogenous Leukemia Revisited". Journal of Clinical Oncology. American Society of Clinical Oncology (ASCO). 19 (11): 2915–2926. doi:10.1200/jco.2001.19.11.2915. ISSN 0732-183X.
- ↑ Druker, Brian J.; Talpaz, Moshe; Resta, Debra J.; Peng, Bin; Buchdunger, Elisabeth; Ford, John M.; Lydon, Nicholas B.; Kantarjian, Hagop; Capdeville, Renaud; Ohno-Jones, Sayuri; Sawyers, Charles L. (April 5, 2001). "Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia". New England Journal of Medicine. Massachusetts Medical Society. 344 (14): 1031–1037. doi: 10.1056/nejm200104053441401 . ISSN 0028-4793.
- ↑ Rebora, P.; Czene, K.; Antolini, L.; Passerini, C. G.; Reilly, M.; Valsecchi, M. G. (September 22, 2010). "Are Chronic Myeloid Leukemia Patients More at Risk for Second Malignancies? A Population-based Study". American Journal of Epidemiology. Oxford University Press (OUP). 172 (9): 1028–1033. doi: 10.1093/aje/kwq262 . ISSN 0002-9262.
- ↑ Gambacorti-Passerini, Carlo; Antolini, Laura; Mahon, François-Xavier; Guilhot, Francois; Deininger, Michael; Fava, Carmen; Nagler, Arnon; Della Casa, Chiara Maria; Morra, Enrica; Abruzzese, Elisabetta; D’Emilio, Anna; Stagno, Fabio; le Coutre, Philipp; Hurtado-Monroy, Rafael; Santini, Valeria; Martino, Bruno; Pane, Fabrizio; Piccin, Andrea; Giraldo, Pilar; Assouline, Sarit; Durosinmi, Muheez A.; Leeksma, Onno; Pogliani, Enrico Maria; Puttini, Miriam; Jang, Eunjung; Reiffers, Josy; Valsecchi, Maria Grazia; Kim, Dong-Wook (March 21, 2011). "Multicenter Independent Assessment of Outcomes in Chronic Myeloid Leukemia Patients Treated With Imatinib". JNCI: Journal of the National Cancer Institute. Oxford University Press (OUP). 103 (7): 553–561. doi: 10.1093/jnci/djr060 . ISSN 1460-2105.
- ↑ Goldman, John M. (2010). "Chronic Myeloid Leukemia: A Historical Perspective". Seminars in Hematology. Elsevier BV. 47 (4): 302–311. doi:10.1053/j.seminhematol.2010.07.001. ISSN 0037-1963.
- ↑ Piazza, Rocco; Valletta, Simona; Winkelmann, Nils; Redaelli, Sara; Spinelli, Roberta; Pirola, Alessandra; Antolini, Laura; Mologni, Luca; Donadoni, Carla; Papaemmanuil, Elli; Schnittger, Susanne; Kim, Dong-Wook; Boultwood, Jacqueline; Rossi, Fabio; Gaipa, Giuseppe; De Martini, Greta P; di Celle, Paola Francia; Jang, Hyun Gyung; Fantin, Valeria; Bignell, Graham R; Magistroni, Vera; Haferlach, Torsten; Pogliani, Enrico Maria; Campbell, Peter J; Chase, Andrew J; Tapper, William J; Cross, Nicholas C P; Gambacorti-Passerini, Carlo (December 9, 2012). "Recurrent SETBP1 mutations in atypical chronic myeloid leukemia". Nature Genetics. Springer Science and Business Media LLC. 45 (1): 18–24. doi:10.1038/ng.2495. ISSN 1061-4036. PMC 3588142 .