
Limestone is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of CaCO3. Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life.

Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses.

Calcium carbonate is a chemical compound with the chemical formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.

The lysocline is the depth in the ocean dependent upon the carbonate compensation depth (CCD), usually around 5 km, below which the rate of dissolution of calcite increases dramatically because of a pressure effect. While the lysocline is the upper bound of this transition zone of calcite saturation, the CCD is the lower bound of this zone.

Aragonite is a carbonate mineral and one of the three most common naturally occurring crystal forms of calcium carbonate, the others being calcite and vaterite. It is formed by biological and physical processes, including precipitation from marine and freshwater environments.

A speleothem is a geological formation by mineral deposits that accumulate over time in natural caves. Speleothems most commonly form in calcareous caves due to carbonate dissolution reactions. They can take a variety of forms, depending on their depositional history and environment. Their chemical composition, gradual growth, and preservation in caves make them useful paleoclimatic proxies.

Ooids are small, spheroidal, "coated" (layered) sedimentary grains, usually composed of calcium carbonate, but sometimes made up of iron- or phosphate-based minerals. Ooids usually form on the sea floor, most commonly in shallow tropical seas. After being buried under additional sediment, these ooid grains can be cemented together to form a sedimentary rock called an oolite. Oolites usually consist of calcium carbonate; these belong to the limestone rock family. Pisoids are similar to ooids, but are larger than 2 mm in diameter, often considerably larger, as with the pisoids in the hot springs at Carlsbad in the Czech Republic.

Dolomite (also known as dolomite rock, dolostone or dolomitic rock) is a sedimentary carbonate rock that contains a high percentage of the mineral dolomite, CaMg(CO3)2. It occurs widely, often in association with limestone and evaporites, though it is less abundant than limestone and rare in Cenozoic rock beds (beds less than about 66 million years in age). One of the first geologists to distinguish dolomite from limestone was Déodat Gratet de Dolomieu; a French mineralogist and geologist whom it is named after. He recognized and described the distinct characteristics of dolomite in the late 18th century, differentiating it from limestone.

Carbonate rocks are a class of sedimentary rocks composed primarily of carbonate minerals. The two major types are limestone, which is composed of calcite or aragonite (different crystal forms of CaCO3), and dolomite rock (also known as dolostone), which is composed of mineral dolomite (CaMg(CO3)2). They are usually classified based on texture and grain size. Importantly, carbonate rocks can exist as metamorphic and igneous rocks, too. When recrystallized carbonate rocks are metamorphosed, marble is created. Rare igneous carbonate rocks even exist as intrusive carbonatites and, even rarer, there exists volcanic carbonate lava.
The carbonate compensation depth (CCD) is the depth, in the oceans, at which the rate of supply of calcium carbonates matches the rate of solvation. That is, solvation 'compensates' supply. Below the CCD solvation is faster, so that carbonate particles dissolve and the carbonate shells (tests) of animals are not preserved. Carbonate particles cannot accumulate in the sediments where the sea floor is below this depth.

Beachrock is a friable to well-cemented sedimentary rock that consists of a variable mixture of gravel-, sand-, and silt-sized sediment that is cemented with carbonate minerals and has formed along a shoreline. Depending on location, the sediment that is cemented to form beachrock can consist of a variable mixture of shells, coral fragments, rock fragments of different types, and other materials. It can contain scattered artifacts, pieces of wood, and coconuts. Beachrock typically forms within the intertidal zone within tropical or semitropical regions. However, Quaternary beachrock is also found as far north and south as 60° latitude.

A calcite sea is a sea in which low-magnesium calcite is the primary inorganic marine calcium carbonate precipitate. An aragonite sea is the alternate seawater chemistry in which aragonite and high-magnesium calcite are the primary inorganic carbonate precipitates. The Early Paleozoic and the Middle to Late Mesozoic oceans were predominantly calcite seas, whereas the Middle Paleozoic through the Early Mesozoic and the Cenozoic are characterized by aragonite seas.

An aragonite sea contains aragonite and high-magnesium calcite as the primary inorganic calcium carbonate precipitates. The chemical conditions of the seawater must be notably high in magnesium content relative to calcium for an aragonite sea to form. This is in contrast to a calcite sea in which seawater low in magnesium content relative to calcium favors the formation of low-magnesium calcite as the primary inorganic marine calcium carbonate precipitate.

A brief, easy-to-understand description of cementation is that minerals bond grains of sediment together by growing around them. This process is called cementation and is a part of the rock cycle.

Micrite is a limestone constituent formed of calcareous particles ranging in diameter up to four μm formed by the recrystallization of lime mud.

Shallow water marine environment refers to the area between the shore and deeper water, such as a reef wall or a shelf break. This environment is characterized by oceanic, geological and biological conditions, as described below. The water in this environment is shallow and clear, allowing the formation of different sedimentary structures, carbonate rocks, coral reefs, and allowing certain organisms to survive and become fossils.

Shell growth in estuaries is an aspect of marine biology that has attracted a number of scientific research studies. Many groups of marine organisms produce calcified exoskeletons, commonly known as shells, hard calcium carbonate structures which the organisms rely on for various specialized structural and defensive purposes. The rate at which these shells form is greatly influenced by physical and chemical characteristics of the water in which these organisms live. Estuaries are dynamic habitats which expose their inhabitants to a wide array of rapidly changing physical conditions, exaggerating the differences in physical and chemical properties of the water.
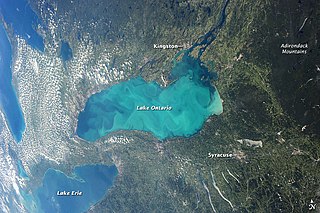
A whiting event is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or sediment disturbance. The phenomenon gets its name from the white, chalky color it imbues to the water. These events have been shown to occur in temperate waters as well as tropical ones, and they can span for hundreds of meters. They can also occur in both marine and freshwater environments. The origin of whiting events is debated among the scientific community, and it is unclear if there is a single, specific cause. Generally, they are thought to result from either bottom sediment re-suspension or by increased activity of certain microscopic life such as phytoplankton. Because whiting events affect aquatic chemistry, physical properties, and carbon cycling, studying the mechanisms behind them holds scientific relevance in various ways.

Marine biogenic calcification is the production of calcium carbonate by organisms in the global ocean.

Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is sometimes called suspended inorganic carbon. In operational terms, it is defined as the inorganic carbon in particulate form that is too large to pass through the filter used to separate dissolved inorganic carbon.





















