Related Research Articles

Anemonia sulcata, or Mediterranean snakelocks sea anemone, is a species of sea anemone in the family Actiniidae from the Mediterranean Sea. Whether A. sulcata should be recognized as a synonym of A. viridis remains a matter of dispute.

Sea anemone neurotoxin is the name given to neurotoxins produced by sea anemones with related structure and function. Sea anemone neurotoxins can be divided in two functional groups that either specifically target the sodium channel or the potassium channel.
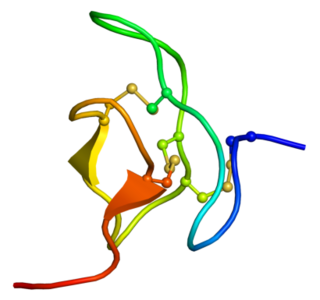
Phrixotoxins are peptide toxins derived from the venom of the Chilean copper tarantula Phrixotrichus auratus, also named Paraphysa scrofa. Phrixotoxin-1 and -2 block A-type voltage-gated potassium channels; phrixotoxin-3 blocks voltage-gated sodium channels. Similar toxins are found in other species, for instance the Chilean rose tarantula.
AETX refers to a group of polypeptide neurotoxins isolated from the sea anemone Anemonia erythraea that target ion channels, altering their function. Four subtypes have been identified: AETX I, II, III and K, which vary in their structure and target.
Jingzhaotoxin proteins are part of a venom secreted by Chilobrachys jingzhao, the Chinese tarantula. and act as neurotoxins. There are several subtypes of jingzhaotoxin, which differ in terms of channel selectivity and modification characteristics. All subspecies act as gating modifiers of sodium channels and/or, to a lesser extent, potassium channels.

Heteroscodratoxin-1 is a neurotoxin produced by the venom glands of Heteroscodra maculata that shifts the activation threshold of voltage-gated potassium channels and the inactivation of Nav1.1 sodium channels to more positive potentials.
Halcurin is a polypeptide neurotoxin from the sea anemone Halcurias sp. Based on sequence homology to type 1 and type 2 sea anemone toxins it is thought to delay channel inactivation by binding to the extracellular site 3 on the voltage gated sodium channels in a membrane potential-dependent manner.
Calitoxin, also known as CLX, is a sea anemone neurotoxin produced by the sea anemone Calliactis parasitica. It targets crabs and octopuses, among other invertebrates. Two isoforms have been identified, both of which are formed from precursors stored in the stinging cells of the anemone. Once the toxin is activated and released, it causes paralysis by increasing neurotransmitter release at invertebrate neuromuscular junctions. Along with several other toxins derived from anemones, CLX is useful in ion channel research. Certain structural aspects of calitoxin are dissimilar from sea anemone toxins that also target the sodium ion channels. Other toxins resembling calitoxin function in completely different ways.
Cangitoxin, also known as CGTX or CGX, is a toxin purified from the venom of the sea anemone Bunodosoma cangicum, which most likely acts by prolonging the inactivation of voltage-gated sodium channels.
CgNa is a peptide toxin isolated from the sea anemone Condylactis gigantea. It causes an increased action potential duration by slowing down the inactivation of tetrodotoxin-sensitive sodium channels.
Kaliseptine (AsKS) is a neurotoxin which can be found in the snakelocks anemone Anemonia viridis. It belongs to a class of sea anemone neurotoxins that inhibits voltage-gated potassium channels.

Antillatoxin (ATX) is a potent lipopeptide neurotoxin produced by the marine cyanobacterium Lyngbya majuscula. ATX activates voltage-gated sodium channels, which can cause cell depolarisation, NMDA-receptor overactivity, excess calcium influx and neuronal necrosis.
Kalicludine (AsKC) is a blocker of the voltage-dependent potassium channel Kv1.2 found in the snakeslocks anemone Anemonia viridis, which it uses to paralyse prey.
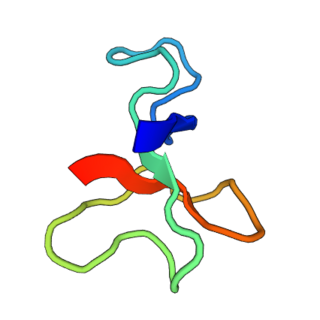
BcIII is a polypeptide sea anemone neurotoxin isolated from Bunodosoma caissarum. It targets the site 3 of voltage-gated sodium channels, thus mainly prolonging the inactivation time course of the channel.
ATX-II, also known as neurotoxin 2, Av2, Anemonia viridis toxin 2 or δ-AITX-Avd1c, is a neurotoxin derived from the venom of the sea anemone Anemonia sulcata. ATX-II slows down the inactivation of different voltage-gated sodium channels, including Nav1.1 and Nav1.2, thus prolonging action potentials.
APETx1 is a peptide toxin from the venom of the sea anemone Anthopleura elegantissima. The toxin acts as a gating modifier on the human ether-à-go-go-related gene (hERG) channel, a type of voltage-gated potassium channel, and as a blocker of voltage-gated sodium channels, including Nav1.2 and Nav1.8.
LmαTX5 is an α-scorpion toxin which inhibits the fast inactivation of voltage-gated sodium channels. It has been identified through transcriptome analysis of the venom gland of Lychas mucronatus, also known as the Chinese swimming scorpion – a scorpion species which is widely distributed in Southeast Asia.

Delta hexatoxin Hv1 is a neurotoxic component found in the venom of the Australian funnel web spider.
AsKC11 is a toxin found in the venom of the sea anemone, Anemonia sulcata. This toxin is part of the Kunitz peptide family and has been shown to be an activator of G protein-coupled inwardly-rectifying potassium (GIRK) channels 1/2, involved in the regulation of cellular excitability.
Kunitz-type serine protease inhibitor APEKTx1 is a peptide toxin derived from the sea anemone Anthopleura elegantissima. This toxin has a dual function, acting both as a serine protease inhibitor and as a selective and potent pore blocker of Kv1.1, a shaker related voltage-gated potassium channel.
References
- 1 2 3 "UniProtKB - P11494 (BDS1_ANESU)".
- 1 2 3 4 5 Yeung, Shuk Yin M.; et al. (2005). "Modulation of Kv3 subfamily potassium currents by the sea anemone toxin BDS: significance for CNS and biophysical studies". Journal of Neuroscience. 25 (38): 8735–45. doi:10.1523/JNEUROSCI.2119-05.2005. PMC 1314979 . PMID 16177043.
- 1 2 3 Liu, Pin; Sooyeon Jo & Bruce P. Bean (2012). "Modulation of neuronal sodium channels by the sea anemone peptide BDS-I". Journal of Neurophysiology. 107 (11): 3155–67. doi:10.1152/jn.00785.2011. PMC 3378363 . PMID 22442564.
- 1 2 Diochot, Sylvie; et al. (1998). "Sea anemone peptides with a specific blocking activity against the fast inactivating potassium channel Kv3. 4". Journal of Biological Chemistry. 273 (12): 6744–9. doi: 10.1074/jbc.273.12.6744 . PMID 9506974.
- ↑ Frazão, Bárbara; Vitor Vasconcelos & Agostinho Antunes (2012). "Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: an overview". Marine Drugs. 10 (12): 1812–51. doi: 10.3390/md10081812 . PMC 3447340 . PMID 23015776.
- 1 2 Driscoll, Paul C.; et al. (1989). "A proton nuclear magnetic resonance study of the antihypertensive and antiviral protein BDS-I from the sea anemone Anemonia sulcata: sequential and stereospecific resonance assignment and secondary structure". Biochemistry. 28 (5): 2178–87. doi:10.1021/bi00431a032. PMID 2566325.