
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets. Most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.

A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions.

IRAK-4, in the IRAK family, is a protein kinase involved in signaling innate immune responses from Toll-like receptors. It also supports signaling from T-cell receptors. IRAK4 contains domain structures which are similar to those of IRAK1, IRAK2, IRAK3 and Pelle. IRAK4 is unique compared to IRAK1, IRAK2 and IRAKM in that it functions upstream of the other IRAKs, but is more similar to Pelle in this trait. IRAK4 has important clinical applications.

Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode receptor tyrosine kinase proteins. Receptor tyrosine kinases have been shown not only to be key regulators of normal cellular processes but also to have a critical role in the development and progression of many types of cancer. Mutations in receptor tyrosine kinases lead to activation of a series of signalling cascades which have numerous effects on protein expression. The receptors are generally activated by dimerization and substrate presentation. Receptor tyrosine kinases are part of the larger family of protein tyrosine kinases, encompassing the receptor tyrosine kinase proteins which contain a transmembrane domain, as well as the non-receptor tyrosine kinases which do not possess transmembrane domains.
A protein kinase inhibitor (PKI) is a type of enzyme inhibitor that blocks the action of one or more protein kinases. Protein kinases are enzymes that phosphorylate (add a phosphate, or PO4, group) to a protein and can modulate its function.

Frizzled is a family of atypical G protein-coupled receptors that serve as receptors in the Wnt signaling pathway and other signaling pathways. When activated, Frizzled leads to activation of Dishevelled in the cytosol.

Dual specificity protein phosphatase 1 is an enzyme that in humans is encoded by the DUSP1 gene.

Protein tyrosine kinase 2 beta is an enzyme that in humans is encoded by the PTK2B gene.
In enzymology, a [tyrosine 3-monooxygenase] kinase is an enzyme that catalyzes the chemical reaction

Discoidin domain receptor family, member 1, also known as DDR1 or CD167a, is a human gene.
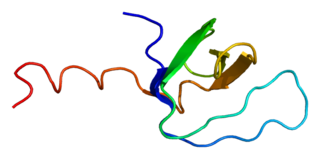
Tyrosine-protein kinase Tec is a tyrosine kinase that in humans is encoded by the TEC gene. Tec kinase is expressed in hematopoietic, liver, and kidney cells and plays an important role in T-helper cell processes. Tec kinase is the name-giving member of the Tec kinase family, a family of non-receptor protein-tyrosine kinases.

Macrophage-stimulating protein receptor is a protein that in humans is encoded by the MST1R gene. MST1R is also known as RON kinase, named after the French city in which it was discovered. It is related to the c-MET receptor tyrosine kinase.

Proto-oncogene tyrosine-protein kinase FER is an enzyme that in humans is encoded by the FER gene.

Protein phosphorylation is a reversible post-translational modification of proteins in which an amino acid residue is phosphorylated by a protein kinase by the addition of a covalently bound phosphate group. Phosphorylation alters the structural conformation of a protein, causing it to become activated, deactivated, or otherwise modifying its function. Approximately 13,000 human proteins have sites that are phosphorylated.

Tyrosine-protein kinase STYK1 is an enzyme that in humans is encoded by the STYK1 gene.
A non-receptor tyrosine kinase (nRTK) is a cytosolic enzyme that is responsible for catalysing the transfer of a phosphate group from a nucleoside triphosphate donor, such as ATP, to tyrosine residues in proteins. Non-receptor tyrosine kinases are a subgroup of protein family tyrosine kinases, enzymes that can transfer the phosphate group from ATP to a tyrosine residue of a protein (phosphorylation). These enzymes regulate many cellular functions by switching on or switching off other enzymes in a cell.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.
The capsule biosynthesis, or CPS operon, is a section of the genome present in some Escherichia coli, of which regulates the production of polysaccharides making up the bacterial capsule. These polysaccharides help protect the bacteria from harsh environments, toxic chemicals, and bacteriophages.
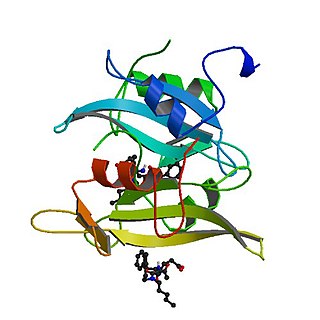
Tyrosine-protein kinase CSK also known as C-terminal Src kinase is an enzyme that, in humans, is encoded by the CSK gene. This enzyme phosphorylates tyrosine residues located in the C-terminal end of Src-family kinases (SFKs) including SRC, HCK, FYN, LCK, LYN and YES1.












