
Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasis and seborrheic dermatitis (dandruff). It may be used in combination with ultraviolet light therapy. Industrially it is a railroad tie preservative and used in the surfacing of roads. Coal tar was listed as a known human carcinogen in the first Report on Carcinogens from the U.S. Federal Government, issued in 1980.
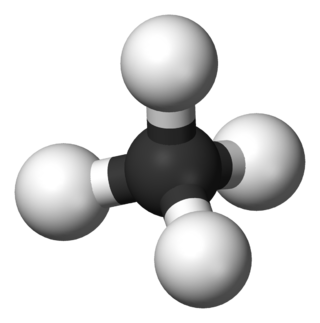
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.

Smoke is a suspension of airborne particulates and gases emitted when a material undergoes combustion or pyrolysis, together with the quantity of air that is entrained or otherwise mixed into the mass. It is commonly an unwanted by-product of fires, but may also be used for pest control (fumigation), communication, defensive and offensive capabilities in the military, cooking, or smoking. It is used in rituals where incense, sage, or resin is burned to produce a smell for spiritual or magical purposes. It can also be a flavoring agent and preservative.

Soot is a mass of impure carbon particles resulting from the incomplete combustion of hydrocarbons. Most broadly, the term includes all the particulate matter produced by this process, including black carbon and residual pyrolysed fuel particles such as coal, cenospheres, charred wood, and petroleum coke classified as cokes or char. It can include polycyclic aromatic hydrocarbons and heavy metals like mercury.

A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are found in coal and in oil deposits, and are also produced by the incomplete combustion of organic matter—for example, in engines and incinerators or when biomass burns in forest fires.

Mycoremediation is a form of bioremediation in which fungi-based remediation methods are used to decontaminate the environment. Fungi have been proven to be a cheap, effective and environmentally sound way for removing a wide array of contaminants from damaged environments or wastewater. These contaminants include heavy metals, organic pollutants, textile dyes, leather tanning chemicals and wastewater, petroleum fuels, polycyclic aromatic hydrocarbons, pharmaceuticals and personal care products, pesticides and herbicides in land, fresh water, and marine environments.
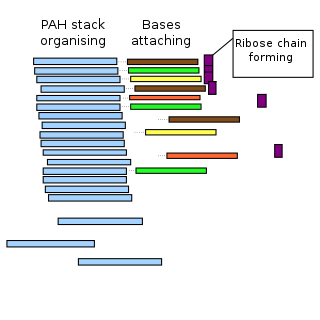
The PAH world hypothesis is a speculative hypothesis that proposes that polycyclic aromatic hydrocarbons (PAHs), known to be abundant in the universe, including in comets, and assumed to be abundant in the primordial soup of the early Earth, played a major role in the origin of life by mediating the synthesis of RNA molecules, leading into the RNA world. However, as yet, the hypothesis is untested.
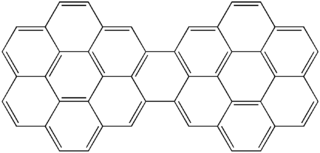
Dicoronylene is the trivial name for a very large polycyclic aromatic hydrocarbon. It has 15 rings and is a brick-red solid. Its formula is C
48H
20. Dicoronylene sublimes under high vacuum, 0.001 torr, between 250 °C and 300 °C.
Chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) are a group of compounds comprising polycyclic aromatic hydrocarbons with two or more aromatic rings and one or more chlorine atoms attached to the ring system. Cl-PAHs can be divided into two groups: chloro-substituted PAHs, which have one or more hydrogen atoms substituted by a chlorine atom, and chloro-added Cl-PAHs, which have two or more chlorine atoms added to the molecule. They are products of incomplete combustion of organic materials. They have many congeners, and the occurrences and toxicities of the congeners differ. Cl-PAHs are hydrophobic compounds and their persistence within ecosystems is due to their low water solubility. They are structurally similar to other halogenated hydrocarbons such as polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and polychlorinated biphenyls (PCBs). Cl-PAHs in the environment are strongly susceptible to the effects of gas/particle partitioning, seasonal sources, and climatic conditions.

A benzopyrene is an organic compound with the formula C20H12. Structurally speaking, the colorless isomers of benzopyrene are pentacyclic hydrocarbons and are fusion products of pyrene and a phenylene group. Two isomeric species of benzopyrene are benzo[a]pyrene and the less common benzo[e]pyrene. They belong to the chemical class of polycyclic aromatic hydrocarbons.

Benzo[j]fluoranthene (BjF) is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it is a colourless solid that is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzo[a]fluoranthene (BaF), benzo[b]fluoranthene (BbF), benzo[e]fluoranthene (BeF), and benzo[k]fluoranthene (BkF). BjF is present in fossil fuels and is released during incomplete combustion of organic matter. It has been traced in the smoke of cigarettes, exhaust from gasoline engines, emissions from the combustion of various types of coal and emissions from oil heating, as well as an impurity in some oils such as soybean oil.

An organic mineral is an organic compound in mineral form. An organic compound is any compound containing carbon, aside from some simple ones discovered before 1828. There are three classes of organic mineral: hydrocarbons, salts of organic acids, and miscellaneous. Organic minerals are rare, and tend to have specialized settings such as fossilized cacti and bat guano. Mineralogists have used statistical models to predict that there are more undiscovered organic mineral species than known ones.

2-Methylnaphthalene is a polycyclic aromatic hydrocarbon (PAH).
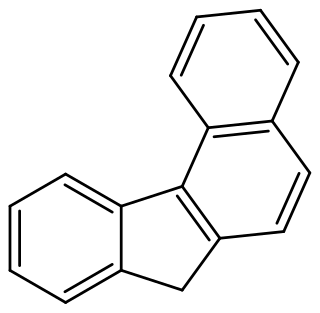
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.
Oil pollution toxicity to marine fish has been observed from oil spills such as the Exxon Valdez disaster, and from nonpoint sources, such as surface runoff, which is the largest source of oil pollution in marine waters.

Passive sampling is an environmental monitoring technique involving the use of a collecting medium, such as a man-made device or biological organism, to accumulate chemical pollutants in the environment over time. This is in contrast to grab sampling, which involves taking a sample directly from the media of interest at one point in time. In passive sampling, average chemical concentrations are calculated over a device's deployment time, which avoids the need to visit a sampling site multiple times to collect multiple representative samples. Currently, passive samplers have been developed and deployed to detect toxic metals, pesticides, pharmaceuticals, radionuclides, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and other organic compounds in water, while some passive samplers can detect hazardous substances in the air.
Occupational hazards of fire debris cleanup are the hazards to health and safety of the personnel tasked with clearing the area of debris and combustion products after a conflagration. Once extinguished, fire debris cleanup poses several safety and health risks for workers. Employers responsible for fire debris cleanup and other work in areas damaged or destroyed by fire are generally obliged by occupational safety and health legislation of the relevant national or regional authority to identify and evaluate hazards, correct any unsafe or unhealthy conditions and provide any necessary training and instruction and personal protective equipment to employees to enable them to carry out the task without undue exposure to hazards. Many of the approaches to control risk in occupational settings can be applied to preventing injuries and disease. This type of work can be completed by general construction firms who may not be fully trained specifically for fire safety and on fire hazards.

Staci Simonich is an American environmental scientist who is a professor and dean for the College of Agricultural Sciences at Oregon State University. Her research considers how chemicals move through the environment. She was appointed Fellow of the American Association for the Advancement of Science in 2021.

Indeno(1,2,3-cd)pyrene is a polycyclic aromatic hydrocarbon (PAH), one of 16 PAHs generally measured in studies of environmental exposure and air pollution. Many compounds of this class are formed when burning coal, oil, gas, wood, household waste and tobacco, and can bind to or form small particles in the air. The compounds are known to have toxic, mutagenic and/or carcinogenic properties. Over 100 different PAHs have been identified in environmental samples, including indeno(1,2,3-cd)pyrene (IP). In 1962, the National Cancer Institute reported that indeno(1,2,3-cd)pyrene has a slight tumor activity. This was confirmed in 1973 by the IARC in mice testing.

















