Related Research Articles

Justus Freiherr von Liebig was a German scientist who made major contributions to agricultural and biological chemistry, and is considered one of the principal founders of organic chemistry. As a professor at the University of Giessen, he devised the modern laboratory-oriented teaching method, and for such innovations, he is regarded as one of the greatest chemistry teachers of all time. He has been described as the "father of the fertilizer industry" for his emphasis on nitrogen and trace minerals as essential plant nutrients, and his popularization of the law of the minimum, which described how plant growth relied on the scarcest nutrient resource, rather than the total amount of resources available. He also developed a manufacturing process for beef extracts, and with his consent a company, called Liebig Extract of Meat Company, was founded to exploit the concept; it later introduced the Oxo brand beef bouillon cube. He popularized an earlier invention for condensing vapors, which came to be known as the Liebig condenser.

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Nitrous oxide, commonly known as laughing gas, nitrous, nitro, or nos, is a chemical compound, an oxide of nitrogen with the formula N
2O. At room temperature, it is a colourless non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful oxidiser similar to molecular oxygen.
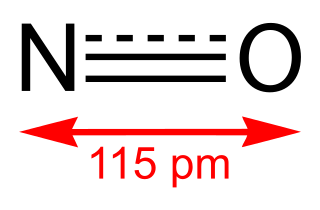
Nitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.
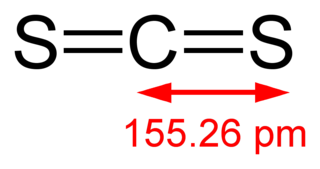
Carbon disulfide is an inorganic compound with the chemical formula CS2 and structure S=C=S. It is a colorless, flammable, neurotoxic liquid that is used as a building block in organic synthesis. Pure carbon disulfide has a pleasant, ether- or chloroform-like odor, but commercial samples are usually yellowish and are typically contaminated with foul-smelling impurities.
The nitrite ion has the chemical formula NO−
2. Nitrite is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid.
Paraldehyde is the cyclic trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane, with a methyl group substituted for a hydrogen atom at each carbon. The corresponding tetramer is metaldehyde. A colourless liquid, it is sparingly soluble in water and highly soluble in ethanol. Paraldehyde slowly oxidizes in air, turning brown and producing an odour of acetic acid. It attacks most plastics and rubbers and should be kept in glass bottles.

Hippuric acid is a carboxylic acid and organic compound. It is found in urine and is formed from the combination of benzoic acid and glycine. Levels of hippuric acid rise with the consumption of phenolic compounds. The phenols are first converted to benzoic acid, and then to hippuric acid and excreted in urine.

Silvering is the chemical process of coating a non-conductive substrate such as glass with a reflective substance, to produce a mirror. While the metal is often silver, the term is used for the application of any reflective metal.
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.

Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry.

"Black snake" is a term that can refer to at least three similar types of fireworks: the Pharaoh's snake, the sugar snake, or a popular retail composition marketed under various product names but usually known as "black snake". The "Pharaoh's snake" or "Pharaoh's serpent" is the original version of the black snake experiment. It produces a more impressive snake, but its execution depends upon mercury (II) thiocyanate, which is no longer in common use due to its toxicity. For a "sugar snake", sodium bicarbonate and sugar are the commonly used chemicals.

The Chugaev elimination is a chemical reaction that involves the elimination of water from alcohols to produce alkenes. The intermediate is a xanthate. It is named for its discoverer, the Russian chemist Lev Aleksandrovich Chugaev (1873-1922), who first reported the reaction sequence in 1899.

Walter Julius Reppe was a German chemist. He is notable for his contributions to the chemistry of acetylene.
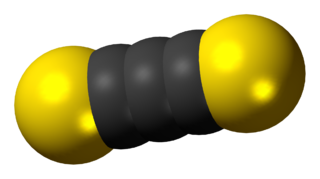
Carbon subsulfide is an organic, sulfur-containing chemical compound with the formula C3S2 and structure S=C=C=C=S. This deep red liquid is immiscible with water but soluble in organic solvents. It readily polymerizes at room temperature to form a hard black solid.

Károly Antal Than de Apát – also called as Carl von Than – was a Hungarian chemist who discovered carbonyl sulfide in 1867.

In chemistry, the haloform reaction is a chemical reaction in which a haloform is produced by the exhaustive halogenation of an acetyl group, in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce chloroform, bromoform, or iodoform. Note that fluoroform can't be prepared in this way.

A kaliapparat is a laboratory device invented in 1831 by Justus von Liebig (1803–1873) for the analysis of carbon in organic compounds. The device, made of glass, consists of a series of five bulbs connected and arranged in a triangular shape.
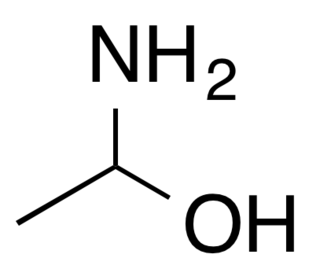
1-Aminoethanol is an organic compound with the formula CH3CH(NH2)OH. It is classified as an alkanolamine. Specifically, it is a structural isomer of 2-aminoethanol (ethanolamine). These two compounds differ in the position of the amino group. Since the central carbon atom in 1-aminoethanol has four different substituents, the compound has two stereoisomers. Unlike 2-aminoethanol, which is of considerable importance in commerce, 1-aminoethanol is not encountered as a pure material and is mainly of theoretical interest.

Urine patches in cattle pastures generate large concentrations of the greenhouse gas nitrous oxide through nitrification and denitrification processes in urine-contaminated soils. Over the past few decades, the cattle population has increased more rapidly than the human population. Between the years 2000 and 2050, the cattle population is expected to increase from 1.5 billion to 2.6 billion. When large populations of cattle are packed into pastures, excessive amounts of urine soak into soils. This increases the rate at which nitrification and denitrification occur and produce nitrous oxide. Currently, nitrous oxide is one of the single most important ozone-depleting emissions and is expected to remain the largest throughout the 21st century.
References
- ↑ Seabourne, Ché Royce; Maxwell, George; Wallace, James (2006). "Taming the Barking Dog". Journal of Chemical Education. 83 (5): 751. Bibcode:2006JChEd..83..751S. doi:10.1021/ed083p751.
- ↑ Brock, William H. (1997). Justus Von Liebig: The Chemical Gatekeeper. Cambridge, England: Cambridge University Press. p. 111. ISBN 9780521524735.
- ↑ Volhard, Jakob (1909). Justus von Liebig, vol. 2 (in German). Liebig, Germany: Johann Ambrosius Barth. pp. 349–350.