
A metallocene is a compound typically consisting of two cyclopentadienyl anions (C
5H−
5, abbreviated Cp) bound to a metal center (M) in the oxidation state II, with the resulting general formula (C5H5)2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride. Certain metallocenes and their derivatives exhibit catalytic properties, although metallocenes are rarely used industrially. Cationic group 4 metallocene derivatives related to [Cp2ZrCH3]+ catalyze olefin polymerization.
Ferrocene is an organometallic compound with the formula Fe(C
5H
5)
2. The molecule consists of two cyclopentadienyl rings bound on opposite sides of a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C
5H
5)+
2.

Terpyridine is a heterocyclic compound derived from pyridine. It is a white solid that is soluble in most organic solvents. The compound is mainly used as a ligand in coordination chemistry.
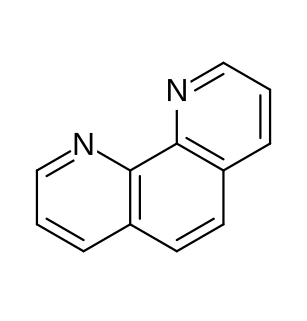
Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. It is used as a ligand in coordination chemistry, forming strong complexes with most metal ions. It is often sold as the monohydrate.

Hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is not clear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reactions in metal complexes are either metal or ligand localized, which is a simplification, albeit a useful one.

Triphos is the name for certain organophosphorus ligands. They are air-sensitive white solids that function as tridentate ligands in coordination and organometallic chemistry.
Organoiron chemistry is the chemistry of iron compounds containing a carbon-to-iron chemical bond. Organoiron compounds are relevant in organic synthesis as reagents such as iron pentacarbonyl, diiron nonacarbonyl and disodium tetracarbonylferrate. Iron adopts oxidation states from Fe(−II) through to Fe(VII). Although iron is generally less active in many catalytic applications, it is less expensive and "greener" than other metals. Organoiron compounds feature a wide range of ligands that support the Fe-C bond; as with other organometals, these supporting ligands prominently include phosphines, carbon monoxide, and cyclopentadienyl, but hard ligands such as amines are employed as well.
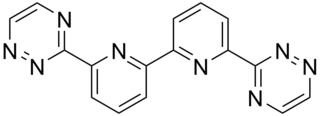
The bis-triazinyl bipyridines (BTBPs) are a class of chemical compounds which are tetradentate ligands similar in shape to quaterpyridine. The BTBPs are made by the reaction of hydrazine and a 1,2-diketone with 6,6'-dicyano-2,2'-bipyridine. The dicyanobipy can be made by reacting 2,2'-bipy with hydrogen peroxide in acetic acid, to form 2,2'-bipyridine-N,N-dioxide. The 2,2'-bipyridine-N,N-dioxide is then converted into the dicyano compound by treatment with potassium cyanide and benzoyl chloride in a mixture of water and THF.
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion (CH
3COCHCOCH−
3) and metal ions, usually transition metals. The bidentate ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have the formula M(acac)3 and M(acac)2. Mixed-ligand complexes, e.g. VO(acac)2, are also numerous. Variations of acetylacetonate have also been developed with myriad substituents in place of methyl (RCOCHCOR′−). Many such complexes are soluble in organic solvents, in contrast to the related metal halides. Because of these properties, acac complexes are sometimes used as catalyst precursors and reagents. Applications include their use as NMR "shift reagents" and as catalysts for organic synthesis, and precursors to industrial hydroformylation catalysts. C
5H
7O−
2 in some cases also binds to metals through the central carbon atom; this bonding mode is more common for the third-row transition metals such as platinum(II) and iridium(III).

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.
Diiminopyridines are a class of diimine ligands. They featuring a pyridine nucleus with imine sidearms appended to the 2,6–positions. The three nitrogen centres bind metals in a tridentate fashion, forming pincer complexes. Diiminopyridines are notable as non-innocent ligand that can assume more than one oxidation state. Complexes of DIPs participate in a range of chemical reactions, including ethylene polymerization, hydrosilylation, and hydrogenation.
Bernard Leslie Shaw, FRS was an English chemist who made notable contributions to organometallic chemistry. He was Professor of Inorganic and Structural Chemistry at the University of Leeds.
Dichlorobis(triphenylphosphine)nickel(II) is a metal phosphine complex with the formula NiCl2[P(C6H5)3]2. It is a dark blue crystalline solid. It is used as a catalyst for organic synthesis.
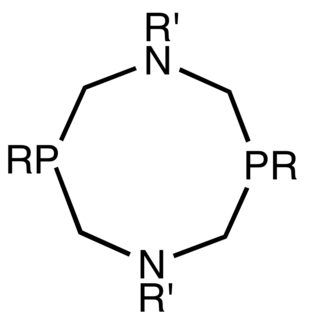
1,5-Diaza-3,7-diphosphacyclooctanes are organophosphorus compounds with the formula [R'NCH2P(R)CH2]2, often abbreviated PR2NR'2. They are air-sensitive, white solids that are soluble in organic solvents. The ligands exist as meso and d,l-diastereomers, but only the meso forms function as bidentate ligands.

Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.

Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted intense scholarly scrutiny. Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc-. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.

Marinella Mazzanti is an Italian inorganic chemist specialized in coordination chemistry. She is a professor at EPFL and the head of the group of Coordination Chemistry at EPFL's School of Basic Sciences.














