Biopanning is an affinity selection technique which selects for peptides that bind to a given target. [1] All peptide sequences obtained from biopanning using combinatorial peptide libraries have been stored in a special freely available database named BDB. [2] [3] This technique is often used for the selection of antibodies too.
Peptides are short chains of amino acid monomers linked by peptide (amide) bonds.
A biological target is anything within a living organism to which some other entity is directed and/or binds, resulting in a change in its behavior or function. Examples of common classes of biological targets are proteins and nucleic acids. The definition is context-dependent, and can refer to the biological target of a pharmacologically active drug compound, the receptor target of a hormone, or some other target of an external stimulus. Biological targets are most commonly proteins such as enzymes, ion channels, and receptors.
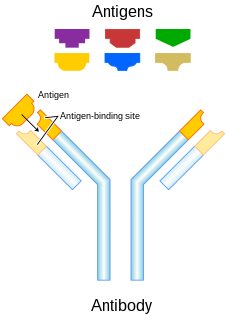
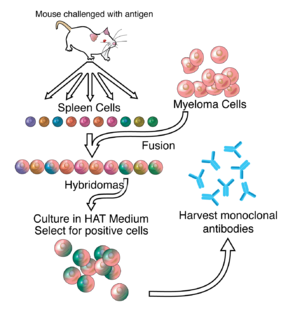
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen, via the Fab's variable region. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize its target directly. Depending on the antigen, the binding may impede the biological process causing the disease or may activate macrophages to destroy the foreign substance. The ability of an antibody to communicate with the other components of the immune system is mediated via its Fc region, which contains a conserved glycosylation site involved in these interactions. The production of antibodies is the main function of the humoral immune system.
Biopanning involves 4 major steps for peptide selection. [4] The first step is to have phage display libraries prepared. This involves inserting foreign desired gene segments into a region of the bacteriophage genome, so that the peptide product will be displayed on the surface of the bacteriophage virion. The most often used are genes pIII or pVIII of bacteriophage M13. [5] The next step is the capturing step. It involves conjugating the phage library to the desired target. This procedure is termed panning. It utilizes the binding interactions so that only specific peptides presented by bacteriophage are bound to the target. For example, selecting antibody presented by bacteriophage with coated antigen in microtiter plates.
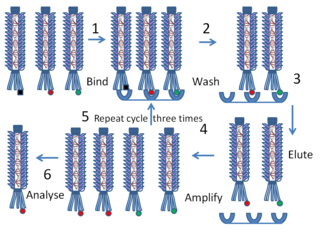
Phage display is a laboratory technique for the study of protein–protein, protein–peptide, and protein–DNA interactions that uses bacteriophages to connect proteins with the genetic information that encodes them. In this technique, a gene encoding a protein of interest is inserted into a phage coat protein gene, causing the phage to "display" the protein on its outside while containing the gene for the protein on its inside, resulting in a connection between genotype and phenotype. These displaying phages can then be screened against other proteins, peptides or DNA sequences, in order to detect interaction between the displayed protein and those other molecules. In this way, large libraries of proteins can be screened and amplified in a process called in vitro selection, which is analogous to natural selection.

In biology, a gene is a sequence of nucleotides in DNA or RNA that codes for a molecule that has a function. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic trait. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes as well as gene–environment interactions. Some genetic traits are instantly visible, such as eye color or number of limbs, and some are not, such as blood type, risk for specific diseases, or the thousands of basic biochemical processes that constitute life.

A bacteriophage, also known informally as a phage, is a virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν (phagein), "to devour". Bacteriophages are composed of proteins that encapsulate a DNA or RNA genome, and may have relatively simple or elaborate structures. Their genomes may encode as few as four genes and as many as hundreds of genes. Phages replicate within the bacterium following the injection of their genome into its cytoplasm. Bacteriophages are among the most common and diverse entities in the biosphere. Bacteriophages are ubiquitous viruses, found wherever bacteria exist. It is estimated there are more than 1031 bacteriophages on the planet, more than every other organism on Earth, including bacteria, combined.
The washing step comes after the capturing step to wash away the unbound phages from solid surface. Only the bound phages with strong affinity are kept. The final step involves the elution step where the bound phages are eluted through changing of pH or other environment conditions.
The end result is the peptides produced by bacteriophage are specific. The resulting filamentous phages can infect gram-negative bacteria once again to produce phage libraries. The cycle can occur many times resulting with strong affinity binding peptides to the target.