Related Research Articles

A zygote is a eukaryotic cell formed by a fertilization event between two gametes. The zygote's genome is a combination of the DNA in each gamete, and contains all of the genetic information of a new individual organism.

An embryo is an initial stage of development of a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male sperm cell. The resulting fusion of these two cells produces a single-celled zygote that undergoes many cell divisions that produce cells known as blastomeres. The blastomeres are arranged as a solid ball that when reaching a certain size, called a morula, takes in fluid to create a cavity called a blastocoel. The structure is then termed a blastula, or a blastocyst in mammals.

A genetic chimerism or chimera is a single organism composed of cells with more than one distinct genotype. In animals and human chimeras, this means an individual derived from two or more zygotes, which can include possessing blood cells of different blood types, subtle variations in form (phenotype) and, if the zygotes were of differing sexes, then even the possession of both female and male sex organs. Animal chimeras are produced by the merger of two embryos. In plant chimeras, however, the distinct types of tissue may originate from the same zygote, and the difference is often due to mutation during ordinary cell division. Normally, genetic chimerism is not visible on casual inspection; however, it has been detected in the course of proving parentage. In contrast, an individual where each cell contains genetic material from two organisms of different breeds, varieties, species or genera is called a hybrid.
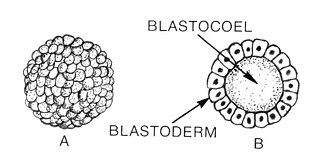
Blastulation is the stage in early animal embryonic development that produces the blastula. In mammalian development the blastula develops into the blastocyst with a differentiated inner cell mass and an outer trophectoderm. The blastula is a hollow sphere of cells known as blastomeres surrounding an inner fluid-filled cavity called the blastocoel. Embryonic development begins with a sperm fertilizing an egg cell to become a zygote, which undergoes many cleavages to develop into a ball of cells called a morula. Only when the blastocoel is formed does the early embryo become a blastula. The blastula precedes the formation of the gastrula in which the germ layers of the embryo form.

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst is reorganized into a multilayered structure known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

The blastocyst is a structure formed in the early embryonic development of mammals. It possesses an inner cell mass (ICM) also known as the embryoblast which subsequently forms the embryo, and an outer layer of trophoblast cells called the trophectoderm. This layer surrounds the inner cell mass and a fluid-filled cavity known as the blastocoel. In the late blastocyst the trophectoderm is known as the trophoblast. The trophoblast gives rise to the chorion and amnion, the two fetal membranes that surround the embryo. The placenta derives from the embryonic chorion and the underlying uterine tissue of the mother.
A germ layer is a primary layer of cells that forms during embryonic development. The three germ layers in vertebrates are particularly pronounced; however, all eumetazoans produce two or three primary germ layers. Some animals, like cnidarians, produce two germ layers making them diploblastic. Other animals such as bilaterians produce a third layer between these two layers, making them triploblastic. Germ layers eventually give rise to all of an animal's tissues and organs through the process of organogenesis.

Embryoid bodies (EBs) are three-dimensional aggregates of pluripotent stem cells.

An organoid is a miniaturized and simplified version of an organ produced in vitro in three dimensions that mimics the key functional, structural and biological complexity of that organ. They are derived from one or a few cells from a tissue, embryonic stem cells or induced pluripotent stem cells, which can self-organize in three-dimensional culture owing to their self-renewal and differentiation capacities. The technique for growing organoids has rapidly improved since the early 2010s, and The Scientist names it as one of the biggest scientific advancements of 2013. Scientists and engineers use organoids to study development and diseases in the laboratory and industry for drug discovery and development, personalized diagnostics and medicine, gene and cell therapies, tissue engineering and regenerative medicine.

The inner cell mass (ICM) or embryoblast is a structure in the early development of an embryo. It is the mass of cells inside the blastocyst that will eventually give rise to the definitive structures of the fetus. The inner cell mass forms in the earliest stages of embryonic development, before implantation into the endometrium of the uterus. The ICM is entirely surrounded by the single layer of trophoblast cells of the trophectoderm.

Growth differentiation factor-3 (GDF3), also known as Vg-related gene 2 (Vgr-2) is protein that in humans is encoded by the GDF3 gene. GDF3 belongs to the transforming growth factor beta (TGF-β) superfamily. It has high similarity to other TGF-β superfamily members including Vg1 and GDF1.

Human embryonic development, or human embryogenesis, is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks has 23 stages.
The pluriblast is a pluripotent population of cells in the embryogenesis of marsupials, called the inner cell mass in eutherians. The pluriblast is distinct from the trophoblast, and gives rise to the germ layers of the embryo, as well as extra embryonic endoderm and extra embryonic mesoderm. Both the pluriblast and trophoblast arise from the totipotent cells of the early conceptus. By definition, the pluriblast does not give rise to trophoblast cells during normal development, although it may retain this potential under experimental conditions.

Janet Rossant, is a developmental biologist well known for her contributions to the understanding of the role of genes in embryo development. She is a world renowned leader in developmental biology. Her current research interests focus on stem cells, molecular genetics, and developmental biology. Specifically, she uses cellular and genetic manipulation techniques to study how genes control both normal and abnormal development of early mouse embryos. Rossant has discovered information on embryo development, how multiple types of stem cells are established, and the mechanisms by which genes control development. In 1998, her work helped lead to the discovery of the trophoblast stem cell, which has assisted in showing how congenital anomalies in the heart, blood vessels, and placenta can occur.

Clemens A. van Blitterswijk is a Dutch tissue engineer who contributed to the use of biomaterials to heal bone injuries, especially using osteoinductive ceramics. In collaboration with Jan de Boer and others, he has contributed to screening microtextures to study cell-biomaterial interactions, an approach termed materiomics.

The Institute of Molecular Biotechnology (IMBA) is an independent biomedical research organisation founded by the Austrian Academy of Sciences in cooperation with the pharmaceutical company Boehringer Ingelheim. The institute employs around 250 people from over 40 countries, who perform basic research. IMBA is located at the Vienna BioCenter (VBC) and shares facilities and scientific training programs with the Gregor Mendel Institute of Molecular Plant Biology (GMI) of the Austrian Academy of Sciences and the Research Institute of Molecular Pathology (IMP), the basic research center of Boehringer Ingelheim.
P19 cells is an embryonic carcinoma cell line derived from an embryo-derived teratocarcinoma in mice. The cell line is pluripotent and can differentiate into cell types of all three germ layers. Also, it is the most characterized embryonic carcinoma (EC) cell line that can be induced into cardiac muscle cells and neuronal cells by different specific treatments. Indeed, exposing aggregated P19 cells to dimethyl sulfoxide (DMSO) induces differentiation into cardiac and skeletal muscle. Also, exposing P19 cells to retinoic acid (RA) can differentiate them into neuronal cells.
The International Society for Stem Cell Research (ISSCR) is an independent 501(c)(3) nonprofit organization based in Evanston, Illinois, United States. The organization's mission is to promote excellence in stem cell science and applications to human health.

Gastruloids are three dimensional aggregates of embryonic stem cells (ESCs) that, when cultured in specific conditions, exhibit an organization resembling that of an embryo. They develop with three orthogonal axes and contain the primordial cells for various tissues derived from the three germ layers, without the presence of extraembryonic tissues. Notably, they do not possess forebrain, midbrain, and hindbrain structures. Gastruloids serve as a valuable model system for studying mammalian development, including human development, as well as diseases associated with it. They are a model system an embryonic organoid for the study of mammalian development and disease.
Valentina Fossati is an Italian stem cell biologist. She is a Senior Research Investigator at the New York Stem Cell Foundation. Her research is focused on developing human stem cell-based models to study the role of glia in neurodegeneration and neuroinflammation.
References
- ↑ Simunovic, Mijo; Brivanlou, Ali H. (14 March 2017). "Embryoids, organoids and gastruloids: new approaches to understanding embryogenesis". Development. 144 (6): 976–985. doi: 10.1242/dev.143529 . PMC 5358114 . PMID 28292844.
- ↑ "Blastoid: The backstory of the formation of blastocyst-like structure solely from stem cells". 27 June 2018.
- ↑ Rivron, Nicolas C.; Frias-Aldeguer, Javier; Vrij, Erik J.; Boisset, Jean-Charles; Korving, Jeroen; Vivié, Judith; Truckenmüller, Roman K.; van Oudenaarden, Alexander; van Blitterswijk, Clemens A.; Geijsen, Niels (May 2018). "Blastocyst-like structures generated solely from stem cells". Nature. 557 (7703): 106–111. Bibcode:2018Natur.557..106R. doi:10.1038/s41586-018-0051-0. PMID 29720634. S2CID 13749109.
- ↑ Rivron, Nicolas C.; Frias-Aldeguer, Javier; Vrij, Erik J.; Boisset, Jean-Charles; Korving, Jeroen; Vivié, Judith; Truckenmüller, Roman K.; van Oudenaarden, Alexander; van Blitterswijk, Clemens A.; Geijsen, Niels (May 2018). "Blastocyst-like structures generated solely from stem cells". Nature. 557 (7703): 106–111. Bibcode:2018Natur.557..106R. doi:10.1038/s41586-018-0051-0. PMID 29720634. S2CID 13749109.
- ↑ Clark, Amander T.; Brivanlou, Ali; Fu, Jianping; Kato, Kazuto; Mathews, Debra; Niakan, Kathy K.; Rivron, Nicolas; Saitou, Mitinori; Surani, Azim; Tang, Fuchou; Rossant, Janet (2021-06-08). "Human embryo research, stem cell-derived embryo models and in vitro gametogenesis: Considerations leading to the revised ISSCR guidelines". Stem Cell Reports. 16 (6): 1416–1424. doi:10.1016/j.stemcr.2021.05.008. ISSN 2213-6711. PMC 8190666 . PMID 34048690.
- ↑ Hyun, Insoo; Munsie, Megan; Pera, Martin F.; Rivron, Nicolas C.; Rossant, Janet (2020-02-11). "Toward Guidelines for Research on Human Embryo Models Formed from Stem Cells". Stem Cell Reports. 14 (2): 169–174. doi:10.1016/j.stemcr.2019.12.008. ISSN 2213-6711. PMC 7015820 . PMID 31951813.
- ↑ Rivron, Nicolas; Pera, Martin; Rossant, Janet; Martinez Arias, Alfonso; Zernicka-Goetz, Magdalena; Fu, Jianping; van den Brink, Susanne; Bredenoord, Annelien; Dondorp, Wybo; de Wert, Guido; Hyun, Insoo (December 2018). "Debate ethics of embryo models from stem cells". Nature. 564 (7735): 183–185. Bibcode:2018Natur.564..183R. doi: 10.1038/d41586-018-07663-9 . ISSN 1476-4687. PMID 30542177. S2CID 54470694.
- ↑ Lovell-Badge, Robin; Anthony, Eric; Barker, Roger A.; Bubela, Tania; Brivanlou, Ali H.; Carpenter, Melissa; Charo, R. Alta; Clark, Amander; Clayton, Ellen; Cong, Yali; Daley, George Q. (2021-06-08). "ISSCR Guidelines for Stem Cell Research and Clinical Translation: The 2021 update". Stem Cell Reports. 16 (6): 1398–1408. doi:10.1016/j.stemcr.2021.05.012. ISSN 2213-6711. PMC 8190668 . PMID 34048692.
- ↑ Fu, Jianping; Warmflash, Aryeh; Lutolf, Matthias P. (February 2021). "Stem-cell-based embryo models for fundamental research and translation". Nature Materials. 20 (2): 132–144. Bibcode:2021NatMa..20..132F. doi:10.1038/s41563-020-00829-9. ISSN 1476-1122. PMC 7855549 . PMID 33199861.