Related Research Articles

Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm to 400 nm, shorter than that of visible light but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionize atoms, it can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules.

A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light. Most materials selectively absorb certain wavelengths of light. Materials that humans have chosen and developed for use as pigments usually have special properties that make them useful for coloring other materials. A pigment must have a high tinting strength relative to the materials it colors. It must be stable in solid form at ambient temperatures.

Ultraviolet–visible spectroscopy or ultraviolet–visible spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible spectral regions. This means it uses light in the visible and adjacent ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, atoms and molecules undergo electronic transitions. Absorption spectroscopy is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.

Dye-sublimation printing is a computer printing technique which uses heat to transfer dye onto materials such as a plastic, card, paper, or fabric. The sublimation name was first applied because the dye was considered to make the transition between the solid and gas states without going through a liquid stage. This understanding of the process was later shown to be incorrect, as there is some liquefying of the dye. Since then, the proper name for the process became to be known as dye-diffusion, though this has not eliminated the original name. Many consumer and professional dye-sublimation printers are designed and used for producing photographic prints, ID cards, clothing, and more.

In chemistry, spectrophotometry is the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. It is more specific than the general term electromagnetic spectroscopy in that spectrophotometry deals with visible light, near-ultraviolet, and near-infrared, but does not cover time-resolved spectroscopic techniques.
Dye penetrant inspection (DP), also called liquid penetrate inspection (LPI) or penetrant testing (PT), is a widely applied and low-cost inspection method used to check surface-breaking defects in all non-porous materials. The penetrant may be applied to all non-ferrous materials and ferrous materials, although for ferrous components magnetic-particle inspection is often used instead for its subsurface detection capability. LPI is used to detect casting, forging and welding surface defects such as hairline cracks, surface porosity, leaks in new products, and fatigue cracks on in-service components.

A label is a piece of paper, plastic film, cloth, metal, or other material affixed to a container or product, on which is written or printed information or symbols about the product or item. Information printed directly on a container or article can also be considered labeling.
Graphite furnace atomic absorption spectroscopy (GFAAS) is a type of spectrometry that uses a graphite-coated furnace to vaporize the sample. Briefly, the technique is based on the fact that free atoms will absorb light at frequencies or wavelengths characteristic of the element of interest. Within certain limits, the amount of light absorbed can be linearly correlated to the concentration of analyte present. Free atoms of most elements can be produced from samples by the application of high temperatures. In GFAAS, samples are deposited in a small graphite or pyrolytic carbon coated graphite tube, which can then be heated to vaporize and atomize the analyte. The atoms absorb ultraviolet or visible light and make transitions to higher electronic energy levels. Applying the Beer-Lambert law directly in AA spectroscopy is difficult due to variations in the atomization efficiency from the sample matrix, and nonuniformity of concentration and path length of analyte atoms. Concentration measurements are usually determined from a working curve after calibrating the instrument with standards of known concentration. The main advantages of the graphite furnace comparing to aspiration atomic absorption are the following:
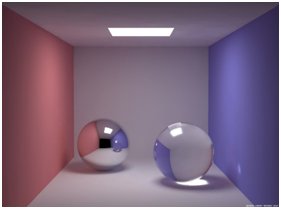
Gloss is an optical property which indicates how well a surface reflects light in a specular (mirror-like) direction. It is one of important parameters that are used to describe the visual appearance of an object. The factors that affect gloss are the refractive index of the material, the angle of incident light and the surface topography.

Sun protective clothing is clothing specifically designed for sun protection and is produced from a fabric rated for its level of ultraviolet (UV) protection. A novel weave structure and denier may produce sun protective properties. In addition, some textiles and fabrics employed in the use of sun protective clothing may be pre-treated with UV-inhibiting ingredients during manufacture to enhance their effectiveness.

Industrial radiography is a method of non-destructive testing where many types of manufactured components can be examined to verify the internal structure and integrity of the specimen. Industrial Radiography can be performed utilizing either X-rays or gamma rays. Both are forms of electromagnetic radiation. The difference between various forms of electromagnetic energy is related to the wavelength. X and gamma rays have the shortest wavelength and this property leads to the ability to penetrate, travel through, and exit various materials such as carbon steel and other metals.
The Platinum-Cobalt Scale is a color scale that was introduced in 1892 by chemist Allen Hazen (1869–1930). The index was developed as a way to evaluate pollution levels in waste water. It has since expanded to a common method of comparison of the intensity of yellow-tinted samples. It is specific to the color yellow and is based on dilutions of a 500 ppm platinum cobalt solution. The colour produced by one milligram of platinum cobalt dissolved in one litre of water is fixed as one unit of colour in platinum-cobalt scale. The ASTM has detailed description and procedures in ASTM Designation D1209, "Standard Test Method for Color of Clear Liquids ".

A color chart or color reference card is a flat, physical object that has many different color samples present. They can be available as a single-page chart, or in the form of swatchbooks or color-matching fans.
Many natural and synthetic polymers are attacked by ultraviolet radiation, and products using these materials may crack or disintegrate if they are not UV-stable. The problem is known as UV degradation, and is a common problem in products exposed to sunlight. Continuous exposure is a more serious problem than intermittent exposure, since attack is dependent on the extent and degree of exposure.
Weather testing of polymers is the controlled polymer degradation and polymer coating degradation under lab or natural conditions.

A glossmeter is an instrument which is used to measure specular reflection gloss of a surface. Gloss is determined by projecting a beam of light at a fixed intensity and angle onto a surface and measuring the amount of reflected light at an equal but opposite angle.
Lightfastness is a property of a colourant such as dye or pigment that describes how resistant to fading it is when exposed to light. Dyes and pigments are used for example for dyeing of fabrics, plastics or other materials and manufacturing paints or printing inks.

An ASTM Smoke Pump, or 'spot pump', is an instrument for evaluating the dark particle concentration of a smoke. It is very widely used (2014) by heating engineers to assess emissions, most commonly from fuel-burning appliances such as a wood stoves or oil boilers. It consists of a cylinder of precise size down which a close-fitting piston is pulled, sucking a sample of the smoke through a small nozzle against a filter paper so that a 'spot' is formed on the paper, the darkness of which can be compared with a standard chart to indicate the concentration of smoke.
Total Base Number (TBN) is a measurement of basicity that is expressed in terms of klk number of milligrams of potassium hydroxide per gram of oil sample. TBN is an important measurement in petroleum products, and the value varies depending on its application. TBN generally ranges from 6–8 mg KOH/g in modern lubricants, 7–10 mg KOH/g for general internal combustion engine use and 10–15 mg KOH/g for diesel engine operations. TBN is typically higher for marine grade lubricants, approximately 15-80 mg KOH/g, as the higher TBN values are designed to increase the operating period under harsh operating conditions, before the lubricant requires replacement.
There are two different types of haze that can occur in materials:
References
- ↑ Textile Fading Card example 1, Gaylord. Retrieved 2010-10-18.
- ↑ Textile Fading Card example 2 Archived July 15, 2011, at the Wayback Machine , Pel. Retrieved 2010-10-18.
- ↑ "Committee D13 on Textiles". www.astm.org. Retrieved 2019-06-03.
- ↑ Faded Card example Archived December 25, 2010, at the Wayback Machine , Talas. Retrieved 2010-10-18.
- ↑ Faded Card example, PDF. Retrieved 2010-10-18.
| This colour-related article is a stub. You can help Wikipedia by expanding it. |