Related Research Articles
Bohrium is a synthetic chemical element; it has symbol Bh and atomic number 107. It is named after Danish physicist Niels Bohr. As a synthetic element, it can be created in particle accelerators but is not found in nature. All known isotopes of bohrium are highly radioactive; the most stable known isotope is 270Bh with a half-life of approximately 2.4 minutes, though the unconfirmed 278Bh may have a longer half-life of about 11.5 minutes.
Meitnerium is a synthetic chemical element; it has symbol Mt and atomic number 109. It is an extremely radioactive synthetic element. The most stable known isotope, meitnerium-278, has a half-life of 4.5 seconds, although the unconfirmed meitnerium-282 may have a longer half-life of 67 seconds. The element was first synthesized in August 1982 by the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany, and it was named after Lise Meitner in 1997.

Neptunium is a chemical element; it has symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. It is named after Neptune, the planet beyond Uranus in the Solar System, which uranium is named after. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. Like all actinides, it is radioactive, poisonous, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous.

Promethium is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in the Earth's crust at any given time. Promethium is one of the only two radioactive elements that are both preceded and followed in the periodic table by elements with stable forms, the other being technetium. Chemically, promethium is a lanthanide. Promethium shows only one stable oxidation state of +3.
Rutherfordium is a synthetic chemical element; it has symbol Rf and atomic number 104. It is named after physicist Ernest Rutherford. As a synthetic element, it is not found in nature and can only be made in a particle accelerator. It is radioactive; the most stable known isotope, 267Rf, has a half-life of about 48 minutes.
Seaborgium is a synthetic chemical element; it has symbol Sg and atomic number 106. It is named after the American nuclear chemist Glenn T. Seaborg. As a synthetic element, it can be created in a laboratory but is not found in nature. It is also radioactive; the most stable known isotopes have half lives on the order of several minutes.

Technetium is a chemical element; it has symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. Technetium and promethium are the only radioactive elements whose neighbours in the sense of atomic number are both stable. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only.
Roentgenium is a synthetic chemical element; it has symbol Rg and atomic number 111. It is extremely radioactive and can only be created in a laboratory. The most stable known isotope, roentgenium-282, has a half-life of 130 seconds, although the unconfirmed roentgenium-286 may have a longer half-life of about 10.7 minutes. Roentgenium was first created in December 1994 by the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany. It is named after the physicist Wilhelm Röntgen, who discovered X-rays. Only a few roentgenium atoms have ever been synthesized, and they have no practical application.
Oganesson is a synthetic chemical element; it has symbol Og and atomic number 118. It was first synthesized in 2002 at the Joint Institute for Nuclear Research (JINR) in Dubna, near Moscow, Russia, by a joint team of Russian and American scientists. In December 2015, it was recognized as one of four new elements by the Joint Working Party of the international scientific bodies IUPAC and IUPAP. It was formally named on 28 November 2016. The name honors the nuclear physicist Yuri Oganessian, who played a leading role in the discovery of the heaviest elements in the periodic table.
Moscovium is a synthetic chemical element; it has symbol Mc and atomic number 115. It was first synthesized in 2003 by a joint team of Russian and American scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia. In December 2015, it was recognized as one of four new elements by the Joint Working Party of international scientific bodies IUPAC and IUPAP. On 28 November 2016, it was officially named after the Moscow Oblast, in which the JINR is situated.
Tennessine is a synthetic chemical element; it has symbol Ts and atomic number 117. It has the second-highest atomic number and joint-highest atomic mass of all known elements and is the penultimate element of the 7th period of the periodic table. It is named after the U.S. state of Tennessee, where key research institutions involved in its discovery are located.
Copernicium is a synthetic chemical element; it has symbol Cn and atomic number 112. Its known isotopes are extremely radioactive, and have only been created in a laboratory. The most stable known isotope, copernicium-285, has a half-life of approximately 30 seconds. Copernicium was first created in February 1996 by the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany. It was named after the astronomer Nicolaus Copernicus on his 537th anniversary.
Flerovium is a synthetic chemical element; it has symbol Fl and atomic number 114. It is an extremely radioactive, superheavy element, named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia, where the element was discovered in 1999. The lab's name, in turn, honours Russian physicist Georgy Flyorov. IUPAC adopted the name on 30 May 2012. The name and symbol had previously been proposed for element 102 (nobelium) but were not accepted by IUPAC at that time.
Unbibium, also known as element 122 or eka-thorium, is a hypothetical chemical element; it has placeholder symbol Ubb and atomic number 122. Unbibium and Ubb are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to follow unbiunium as the second element of the superactinides and the fourth element of the 8th period. Similarly to unbiunium, it is expected to fall within the range of the island of stability, potentially conferring additional stability on some isotopes, especially 306Ubb which is expected to have a magic number of neutrons (184).
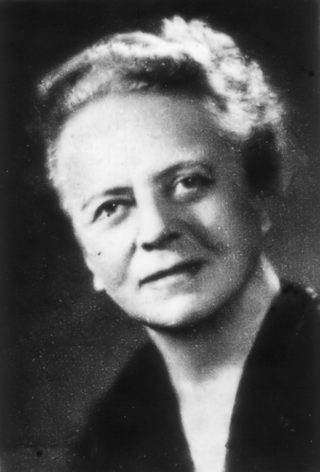
Ida Noddack, néeTacke, was a German chemist and physicist. In 1934 she was the first to mention the idea later named nuclear fission. With her husband Walter Noddack, and Otto Berg, she discovered element 75, rhenium. She was nominated three times for the Nobel Prize in Chemistry.
Ausenium and hesperium were the names initially assigned to the transuranic elements with atomic numbers 93 and 94, respectively. The discovery of the elements, now discredited, was made by Enrico Fermi and a team of scientists at the University of Rome in 1934.
Eugene Theodore Booth, Jr. was an American nuclear physicist. He was a member of the historic Columbia University team which made the first demonstration of nuclear fission in the United States. During the Manhattan Project, he worked on gaseous diffusion for isotope separation. He was the director of the design, construction, and operation project for the 385-Mev synchrocyclotron at the Nevis Laboratories, the scientific director of the SCALANT Research Center, and dean of graduate studies at Stevens Institute of Technology. Booth was the scientific director of the SCALANT Research Center, in Italy.
Unbiunium, also known as eka-actinium or element 121, is a hypothetical chemical element; it has symbol Ubu and atomic number 121. Unbiunium and Ubu are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be the first of the superactinides, and the third element in the eighth period. It has attracted attention because of some predictions that it may be in the island of stability. It is also likely to be the first of a new g-block of elements.
Unbiquadium, also known as element 124 or eka-uranium, is a hypothetical chemical element; it has placeholder symbol Ubq and atomic number 124. Unbiquadium and Ubq are the temporary IUPAC name and symbol, respectively, until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table, unbiquadium is expected to be a g-block superactinide and the sixth element in the 8th period. Unbiquadium has attracted attention, as it may lie within the island of stability, leading to longer half-lives, especially for 308Ubq which is predicted to have a magic number of neutrons (184).

Nuclear fission was discovered in December 1938 by chemists Otto Hahn and Fritz Strassmann and physicists Lise Meitner and Otto Robert Frisch. Fission is a nuclear reaction or radioactive decay process in which the nucleus of an atom splits into two or more smaller, lighter nuclei and often other particles. The fission process often produces gamma rays and releases a very large amount of energy, even by the energetic standards of radioactive decay. Scientists already knew about alpha decay and beta decay, but fission assumed great importance because the discovery that a nuclear chain reaction was possible led to the development of nuclear power and nuclear weapons. Hahn was awarded the 1944 Nobel Prize in Chemistry for the discovery of nuclear fission.
References
- ↑ Stern, A. (1934). "The new element bohemium the origin of proto-actinium". Journal of the Society of Chemical Industry. 53 (31): 678–686. doi:10.1002/jctb.5000533104.
- 1 2 Koblic, Odolen (1934). Chemiker Zeitung. 58: 683.
{{cite journal}}: Missing or empty|title=(help) - ↑ Noddack, Ida (1934). "Über das Element 93". Angewandte Chemie. 47 (37): 653–655. Bibcode:1934AngCh..47..653N. doi:10.1002/ange.19340473707.
- ↑ Speter, M. (1934). "BOHEMIUM—AN OBITUARY". Science. 80 (2086): 588–9. Bibcode:1934Sci....80..588S. doi:10.1126/science.80.2086.588.b. PMID 17798409.
- ↑ Sime, Ruth Lewin (2000). "The Search for Transuranium Elements and the Discovery of Nuclear Fission". Physics in Perspective. 2 (1): 48–62. Bibcode:2000PhP.....2...48S. doi:10.1007/s000160050036. S2CID 117751813.
- ↑ "A New Radioactive Element beyond Uranium". Nature. 134 (3376): 55. 1934. Bibcode:1934Natur.134R..55.. doi: 10.1038/134055b0 .