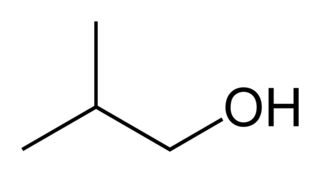
A bradytroph is a strain of an organism that exhibits slow growth in the absence of an external source of a particular metabolite. This is usually due to a defect in an enzyme required in the metabolic pathway producing this chemical. Such defects are the result of mutations in the genes encoding these enzymes. As the organism can still produce small amounts of the chemical, the mutation is not lethal. In these bradytroph strains, rapid growth occurs when the chemical is present in the cell's growth media and the missing metabolite can be transported into the cell from the external environment. A bradytroph may also be referred to as a "leaky auxotroph".

Metabolism is the set of life-sustaining chemical reactions in organisms. The three main purposes of metabolism are: the conversion of food to energy to run cellular processes; the conversion of food/fuel to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of nitrogenous wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments..

Enzymes are macromolecular biological catalysts. Enzymes accelerate chemical reactions. The molecules upon which enzymes may act are called substrates and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called enzymology and a new field of pseudoenzyme analysis has recently grown up, recognising that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties.

In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell. These enzymes often require dietary minerals, vitamins, and other cofactors to function.
The first usage of "bradytroph" was to describe Escherichia coli mutants partially defective in arginine biosynthesis. [1] Among many other examples of bradytrophic strains of microorganisms are Bacillus subtilis strains with mutations affecting thiamine production [2] and Saccharomyces cerevisiae strains with mutations that impair arginine biosynthesis. [3]

Escherichia coli, also known as E. coli, is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus Escherichia that is commonly found in the lower intestine of warm-blooded organisms (endotherms). Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in their hosts, and are occasionally responsible for product recalls due to food contamination. The harmless strains are part of the normal microbiota of the gut, and can benefit their hosts by producing vitamin K2, and preventing colonization of the intestine with pathogenic bacteria, having a symbiotic relationship. E. coli is expelled into the environment within fecal matter. The bacterium grows massively in fresh fecal matter under aerobic conditions for 3 days, but its numbers decline slowly afterwards.

Arginine, also known as L-arginine (symbol Arg or R), is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, an α-carboxylic acid group, and a side chain consisting of a 3-carbon aliphatic straight chain ending in a guanidino group. At physiological pH, the carboxylic acid is deprotonated (−COO−), the amino group is protonated (−NH3+), and the guanidino group is also protonated to give the guanidinium form (-C-(NH2)2+), making arginine a charged, aliphatic amino acid. It is the precursor for the biosynthesis of nitric oxide. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG.

A microorganism, or microbe, is a microscopic organism, which may exist in its single-celled form or in a colony of cells.