
In organic chemistry, isocyanate is the functional group with the formula R−N=C=O. Organic compounds that contain an isocyanate group are referred to as isocyanates. An organic compound with two isocyanate groups is known as a diisocyanate. Diisocyanates are manufactured for the production of polyurethanes, a class of polymers.
Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.
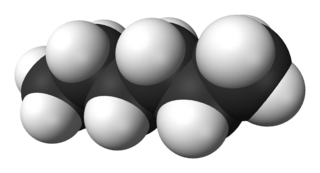
Hexane or n-hexane is an organic compound, a straight-chain alkane with six carbon atoms and the molecular formula C6H14.
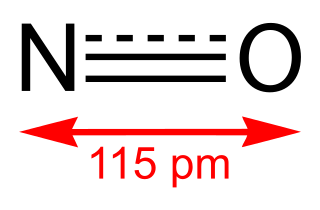
Nitric oxide is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula. Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding.
Chemical Markup Language is an approach to managing molecular information using tools such as XML and Java. It was the first domain specific implementation based strictly on XML, first based on a DTD and later on an XML Schema, the most robust and widely used system for precise information management in many areas. It has been developed over more than a decade by Murray-Rust, Rzepa and others and has been tested in many areas and on a variety of machines.
In a chemistry laboratory a solvent cabinet is a chemical storage cabinet or cupboard which is properly labeled and equipped, for the storage of solvents. A solvent cabinet should be positioned separately from acid cabinet or base cabinet (used for storing acids and caustic bases respectively, as solvents are not compatible with these substances..

Biphenyl is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one hydrogen may use the prefixes xenyl or diphenylyl.

Beryllium fluoride is the inorganic compound with the formula Be F2. This white solid is the principal precursor for the manufacture of beryllium metal. Its structure resembles that of quartz, but BeF2 is highly soluble in water.
Phellandrenes are a pair of organic compounds that have a similar molecular structure and similar chemical properties. α-Phellandrene and β-phellandrene are cyclic monoterpenes and are double-bond isomers. In α-phellandrene, both double bonds are endocyclic and in β-phellandrene, one of them is exocyclic. Both are insoluble in water, but miscible with diethyl ether.
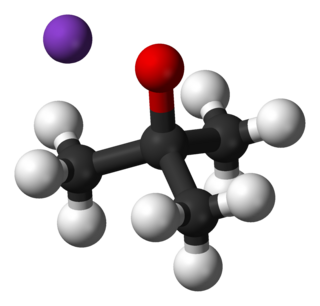
Potassium tert-butoxide (or potassium t-butoxide) is a chemical compound with the formula [(CH3)3COK]n (abbr. KOtBu). This colourless solid is a strong base (pKa of conjugate acid around 17), which is useful in organic synthesis. The compound is often depicted as a salt, and it often behaves as such, but its ionization depends on the solvent.
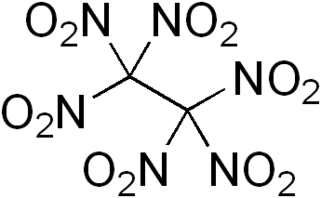
Hexanitroethane (HNE) is an organic compound with chemical formula C2N6O12 or (O2N)3C-C(NO2)3. It is a solid matter with a melting point of 135 °C.

Mercury(II) bromide or mercuric bromide is an inorganic compound with the formula HgBr2. This white solid is a laboratory reagent. Like all mercury salts, it is highly toxic.
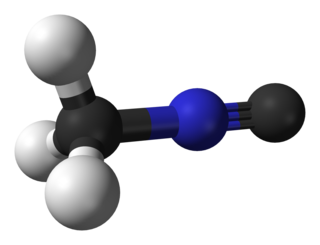
Methyl isocyanide or isocyanomethane is an organic compound and a member of the isocyanide family. This colorless liquid is isomeric and isoelectronic to methyl cyanide (acetonitrile), but its reactivity is very different. In contrast to the faintly sweet, ethereal odor of acetonitrile, the smell of methyl isocyanide, like that of other simple volatile isocyanides, is distinctly penetrating and vile. Methyl isocyanide is mainly used for making 5-membered heterocyclic rings. The C-N distance in methyl isocyanide is very short, 1.158 Å as is characteristic of isocyanides.

Diacetyl peroxide is the organic peroxide with the formula (CH3CO2)2. It is a white solid or oily liquid with a sharp odor. As with a number of organic peroxides, it is explosive. It is often used as a solution, e.g., in dimethyl phthalate.

Methyl azide is an organic compound with the formula CH3N3. It is a white solid and it is the simplest organic azide.
Leslie Bretherick (1926–2003) was a chemist and an “internationally recognized authority on laboratory safety” especially remembered for writing the book which became Bretherick's Handbook of Reactive Chemical Hazards, an indexed guide to published data on dangerous chemical reactions. An obituary said “His contributions have improved the lives of countless millions, and I will aver, have saved the lives of thousands”.

Pentamethyltantalum is a homoleptic organotantalum compound. It has a propensity to explode when it is melted. Its discovery was part of a sequence that led to Richard R. Schrock's Nobel Prize discovery in olefin metathesis.

Iodine azide is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen.

Chromium(II) sulfide is an inorganic compound of chromium and sulfur with the chemical formula CrS. The compound forms black hexagonal crystals, insoluble in water.
Silver chlorite is a chemical compound with the formula AgClO2. This slightly yellow solid is shock sensitive and has an orthorhombic crystal structure.















