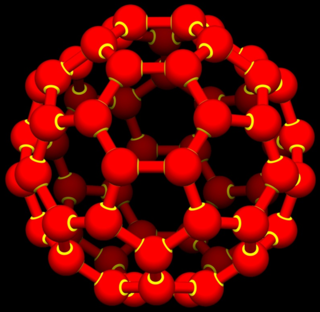
A buckyball or buckminsterfullerene is a molecule resembling a soccer ball composed of 60 carbon atoms.
Buckyball may also refer to:
- Truncated icosahedron, the geometric structure of the C60 molecule
- A brand of neodymium magnet toys
A buckyball or buckminsterfullerene is a molecule resembling a soccer ball composed of 60 carbon atoms.
Buckyball may also refer to:

A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. Graphene, which is a flat mesh of regular hexagonal rings, can be seen as an extreme member of the family.

Sir Harold Walter Kroto, known as Harry Kroto, was an English chemist. He shared the 1996 Nobel Prize in Chemistry with Robert Curl and Richard Smalley for their discovery of fullerenes. He was the recipient of many other honors and awards.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Richard Errett Smalley was an American chemist who was the Gene and Norman Hackerman Professor of Chemistry, Physics, and Astronomy at Rice University. In 1996, along with Robert Curl, also a professor of chemistry at Rice, and Harold Kroto, a professor at the University of Sussex, he was awarded the Nobel Prize in Chemistry for the discovery of a new form of carbon, buckminsterfullerene, also known as buckyballs. He was an advocate of nanotechnology and its applications.

Robert Floyd Curl Jr. was an American chemist who was Pitzer–Schlumberger Professor of Natural Sciences and Professor of Chemistry at Rice University. He was awarded the Nobel Prize in Chemistry in 1996 for the discovery of the nanomaterial buckminsterfullerene, and hence the fullerene class of materials, along with Richard Smalley and Harold Kroto of the University of Sussex.

In geometry, the truncated icosahedron is an Archimedean solid, one of 13 convex isogonal nonprismatic solids whose 32 faces are two or more types of regular polygons. It is the only one of these shapes that does not contain triangles or squares. In general usage, the degree of truncation is assumed to be uniform unless specified.

Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons and twelve pentagons, and resembles a soccer ball. Each of its 60 carbon atoms is bonded to its three neighbors.
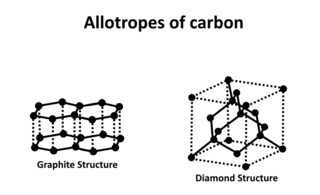
Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).

In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is symmetrical with respect to rotation about the bond axis. By this definition, common forms of sigma bonds are s+s, pz+pz, s+pz and dz2+dz2 . Quantum theory also indicates that molecular orbitals (MO) of identical symmetry actually mix or hybridize. As a practical consequence of this mixing of diatomic molecules, the wavefunctions s+s and pz+pz molecular orbitals become blended. The extent of this mixing depends on the relative energies of the MOs of like symmetry.

In organic chemistry, a Platonic hydrocarbon is a hydrocarbon (molecule) whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed.
Dodecahedrane is a chemical compound, a hydrocarbon with formula C20H20, whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron. Each carbon is bound to three neighbouring carbon atoms and to a hydrogen atom. This compound is one of the three possible Platonic hydrocarbons, the other two being cubane and tetrahedrane.
C60, C.60, or C-60 may refer to:
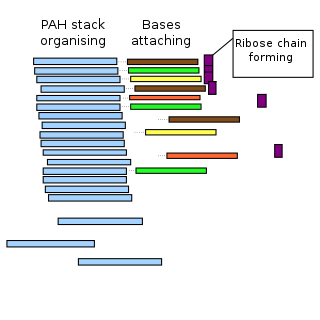
The PAH world hypothesis is a speculative hypothesis that proposes that polycyclic aromatic hydrocarbons (PAHs), known to be abundant in the universe, including in comets, and assumed to be abundant in the primordial soup of the early Earth, played a major role in the origin of life by mediating the synthesis of RNA molecules, leading into the RNA world. However, as yet, the hypothesis is untested.

C70 fullerene is the fullerene molecule consisting of 70 carbon atoms. It is a cage-like fused-ring structure which resembles a rugby ball, made of 25 hexagons and 12 pentagons, with a carbon atom at the vertices of each polygon and a bond along each polygon edge. A related fullerene molecule, named buckminsterfullerene (C60 fullerene), consists of 60 carbon atoms.
Azafullerenes are a class of heterofullerenes in which the element substituting for carbon is nitrogen. They can be in the form of a hollow sphere, ellipsoid, tube, and many other shapes. Spherical azafullerenes resemble the balls used in football (soccer). They are also a member of the carbon nitride class of materials that include beta carbon nitride (β-C3N4), predicted to be harder than diamond. Besides the pioneering work of a couple of academic groups, this class of compounds has so far garnered little attention from the broader fullerene research community. Many properties and structures are yet to be discovered for the highly-nitrogen substituted subset of molecules.
Donald R. Huffman (born 1935) is a Professor Emeritus of Physics at the University of Arizona. With Wolfgang Krätschmer, he developed a technique in 1990 for the simple production of large quantities of C60, or Buckminsterfullerene. Previously, in 1982~1983, he and Krätschmer had found, in a UV spectrum, the first signal of C60 ever observed.
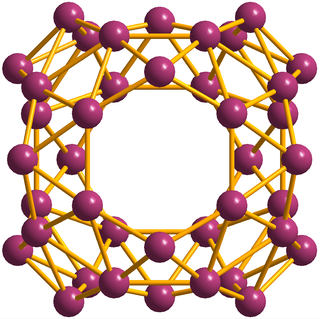
Borospherene (B40) is a cluster molecule containing 40 boron atoms. It is similar to buckminsterfullerene, the "spherical" carbon structure, but with a different symmetry. The discovery of borospherene was announced in July 2014, and is described in the journal Nature Chemistry. Borospherene is similar to other cluster molecules, including buckminsterfullerene (C60), stannaspherene, and plumbaspherene. The molecule includes unusual heptagonal faces.

Volleyballene term refers to a hypothetical chemical compound that is a new type of 3D hollow molecule composed of carbon and transition metals. The name is a reference to fullerenes. The main feature of these substances is that metal atoms are part of the framework and they are not deposited on the surface of the molecule. The incorporation of the metal atoms avoids their clustering and confers to volleyballene sites to attach hydrogen. Volleyballene was predicted in 2016 by Jing Wang et al. A further study based on Density functional Theory (DFT) carried out by Tlahuice-Flores in the same year supports the predicted structure and gives computed Infrared, Raman and UV spectra for its experimental detection. The structure is described as one Sc8 cluster holding 12 scandium atoms linked to six C10 units on each face. The chemical formula C60Sc20 is closely related to C80 fullerene and it has a large predicted HOMO-LUMO gap of 1.47 eV. Further hydrogenation of volleyballene is predicted to give a 70-H structure with an adsorption energy of circa −0.11 eV/H2. Moreover, it is expected that the adsorption-desorption reaction can be reached at ambient temperature. Potential use of volleyballenes is hydrogen storage even at ambient conditions.
Nancy Sarah Goroff is an American organic chemist who is chair of the chemistry department at Stony Brook University. Her research investigates conjugated organic molecules, including polymers, halocarbons and buckyballs. During the 2020 United States elections Goroff ran to represent New York's 1st congressional district, and was defeated by the incumbent, Lee Zeldin.
Lucy Marie Ziurys is an American astrochemist known for her work on high-resolution molecular spectroscopy. She is Regent's Professor of Chemistry & Biology and of Astronomy at the University of Arizona.