
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It functions as a metabolic intermediate in the biosynthesis of the androgen and estrogen sex steroids both in the gonads and in various other tissues. However, DHEA also has a variety of potential biological effects in its own right, binding to an array of nuclear and cell surface receptors, and acting as a neurosteroid and modulator of neurotrophic factor receptors.

Androstenol, also known as 5α-androst-16-en-3α-ol, is a steroidal pheromone and neurosteroid in humans and other mammals, notably pigs. It possesses a characteristic musk-like odor.
3β-Hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD) is an enzyme that catalyzes the biosynthesis of the steroid progesterone from pregnenolone, 17α-hydroxyprogesterone from 17α-hydroxypregnenolone, and androstenedione from dehydroepiandrosterone (DHEA) in the adrenal gland. It is the only enzyme in the adrenal pathway of corticosteroid synthesis that is not a member of the cytochrome P450 family. It is also present in other steroid-producing tissues, including the ovary, testis and placenta. In humans, there are two 3β-HSD isozymes encoded by the HSD3B1 and HSD3B2 genes.
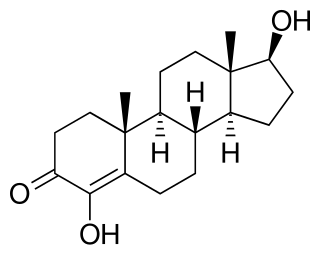
4-Hydroxytestosterone (4-OHT), also known as 4,17β-dihydroxyandrost-4-en-3-one, is a synthetic anabolic-androgenic steroid (AAS) and a derivative of testosterone that was never marketed. It was first patented by G.D. Searle & Company in 1955 and is testosterone with a hydroxy group at the four position. 4-OHT has moderate anabolic, mild androgenic, and anti-aromatase properties and is similar to the steroid clostebol (4-chlorotestosterone).

Epiandrosterone, or isoandrosterone, also known as 3β-androsterone, 3β-hydroxy-5α-androstan-17-one, or 5α-androstan-3β-ol-17-one, is a steroid hormone with weak androgenic activity. It is a metabolite of testosterone and dihydrotestosterone (DHT). It was first isolated in 1931, by Adolf Friedrich Johann Butenandt and Kurt Tscherning. They distilled over 17,000 litres of male urine, from which they got 50 milligrams of crystalline androsterone, which was sufficient to find that the chemical formula was very similar to estrone.

16α-Hydroxydehydroepiandrosterone is an endogenous metabolite of dehydroepiandrosterone (DHEA). Both 16α-OH-DHEA and its 3β-sulfate ester, 16α-OH-DHEA-S, are intermediates in the biosynthesis of estriol from dehydroepiandrosterone (DHEA). 16α-OH-DHEA has estrogenic activity.

4-Dehydroepiandrosterone (4-DHEA) is a steroid that is an isomer of 5-dehydroepiandrosterone.

7-Ketodehydroepiandrosterone (7-keto-DHEA,7-oxo-DHEA), also known as 7-oxoprasterone, is a prohormone produced by metabolism of the prohormone dehydroepiandrosterone (DHEA).

16α-Hydroxydehydroepiandrosterone sulfate (16α-OH-DHEA-S), also known as 16α-hydroxy-17-oxoandrost-5-en-3β-yl sulfate, is an endogenous, naturally occurring steroid and a metabolic intermediate in the production of estriol from dehydroepiandrosterone (DHEA) during pregnancy. It is the C3β sulfate ester of 16α-hydroxy-DHEA.

16α-Hydroxyandrostenedione (16α-OH-A4), also known as 16α-hydroxyandrost-4-ene-3,17-dione, is an endogenous and naturally occurring steroid and metabolic intermediate in the biosynthesis of estriol during pregnancy. It is produced from dehydroepiandrosterone (DHEA), which is converted into 16α-hydroxy-DHEA sulfate, then desulfated and aromatized into 16α-hydroxyestrone, and finally converted into estriol by 17β-hydroxysteroid dehydrogenase.

7β-Hydroxyepiandrosterone (7β-OH-EPIA), also known as 5α-androstan-3β,7β-diol-17-one, is an endogenous androgen, estrogen, and neurosteroid that is produced from dehydroepiandrosterone and epiandrosterone. It has neuroprotective effects and, along with 7α-hydroxyepiandrosterone, may mediate the neuroprotective effects of DHEA. 7β-OH-EPIA may act as a highly potent antagonist of the G protein-coupled estrogen receptor (GPER).

1-Androsterone is a synthetic, orally active anabolic-androgenic steroid (AAS). It is an androgen prohormone of 1-testosterone (dihydroboldenone), 1-androstenedione, and other 1-dehydrogenated androstanes. The drug has been sold on the Internet as a designer steroid and "dietary supplement". It is a positional isomer of dehydroepiandrosterone.
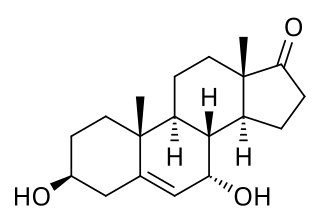
7α-Hydroxydehydroepiandrosterone, also known as 3β,7α-dihydroxyandrost-4-ene-17-one, is an endogenous, naturally occurring steroid and a major metabolite of dehydroepiandrosterone (DHEA) that is formed by CYP7B1 in tissues such as the prostate gland and by CYP3A4 in the liver. The major metabolic pathway of DHEA outside the liver is via 7-hydroxylation into 7α-OH-DHEA and 7β-OH-DHEA. 7α-OH-DHEA has weak estrogenic activity, selectively activating the estrogen receptor ERβ. In addition, 7α-OH-DHEA may be responsible for the known antiglucocorticoid effects of DHEA.
7-Hydroxy-DHEA, or 7-hydroxydehydroepiandrosterone, may refer to:

7β-Hydroxydehydroepiandrosterone, also known as 3β,7β-dihydroxyandrost-4-ene-17-one, is an endogenous, naturally occurring steroid and a metabolite of dehydroepiandrosterone (DHEA). The major metabolic pathway of DHEA outside the liver is via 7-hydroxylation into 7α-OH-DHEA and 7β-OH-DHEA. 7β-OH-DHEA has weak antiestrogenic activity, selectively antagonizing the estrogen receptor ERβ.
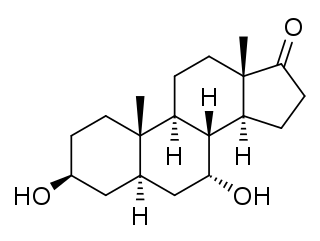
7α-Hydroxyepiandrosterone (7α-OH-EPIA), also known as 3β,7α-dihydroxy-5α-androstan-17-one, is an endogenous, naturally occurring metabolite of epiandrosterone and dehydroepiandrosterone (DHEA) that is formed by the enzyme CYP7B1 in tissues such as the liver and brain.
7-Hydroxyepiandrosterone (7-OH-EPIA) may refer to:

15α-Hydroxydehydroepiandrosterone, abbreviated as 15α-hydroxy-DHEA or 15α-OH-DHEA, is an endogenous metabolite of dehydroepiandrosterone (DHEA). Both 15α-OH-DHEA and its 3β-sulfate ester, 15α-OH-DHEA-S, are intermediates in the biosynthesis of estetrol from dehydroepiandrosterone (DHEA).

15α-Hydroxydehydroepiandrosterone sulfate, abbreviated as 15α-hydroxy-DHEA sulfate or 15α-OH-DHEA-S, also known as 15α-hydroxy-17-oxoandrost-5-en-3β-yl sulfate, is an endogenous, naturally occurring steroid and a metabolic intermediate in the production of estetrol from dehydroepiandrosterone (DHEA) during pregnancy. It is the C3β sulfate ester of 15α-hydroxy-DHEA.















