The molecular formula C4H9N (molar mass: 71.12 g/mol) may refer to:
- Pyrrolidine (azolidine)
- Cyclobutylamine (cyclobutanamine)
- 2,2-Dimethylaziridine
- 1,2-Dimethylaziridine
The molecular formula C4H9N (molar mass: 71.12 g/mol) may refer to:
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.

A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge.
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance in that sample, measured in moles. It is the mass of 1 mole of the substance or 6.022×1023 particles, expressed in grams. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.
Benzol may refer to:

The structural formula of a chemical compound is a graphic representation of the molecular structure, showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike chemical formulas, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same chemical formula.
C2H4O2 may refer to:
The molecular formula C2H6O (molar mass: 46.07 g/mol, exact mass: 46.04186 u) may refer to:
Dichloroethene or dichloroethylene, often abbreviated as DCE, can refer to any one of several isomeric forms of the organochloride with the molecular formula C2H2Cl2:
The molecular formula C6H8O6 (molar mass: 176.124 g/mol) may be:
To see a more detailed description of these isomers, see Amyl alcohol.
The molecular formula C6H14 may refer to:
Dihydroxybenzoic acids (DHBA) are a type of phenolic acids.
Dioxin may refer to:
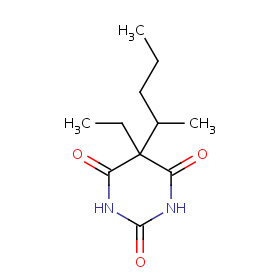
The molecular formula C11H18N2O3 (molar mass: 226.27 g/mol) may be referred as:
Dihydroxycinnamic acid may refer to several molecules with the molecular formula C9H8O4 including:
Octynes are alkynes with one triple bond and the molecular formula C8H14.
Nonynes are alkynes with one triple bond and the molecular formula C9H16.
Decynes are alkynes with one triple bond and the molecular formula C10H18.
Benzoin may refer to:
Heptynes are alkynes with one triple bond and the molecular formula C7H12.