
C5H10 is the molecular formula' of 13 hydrocarbon isomers (represented by their CAS numbers on the chart). They can be divided into cycloalkanes and alkenes.

C5H10 is the molecular formula' of 13 hydrocarbon isomers (represented by their CAS numbers on the chart). They can be divided into cycloalkanes and alkenes.

In chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.
1,3-Butadiene is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene.
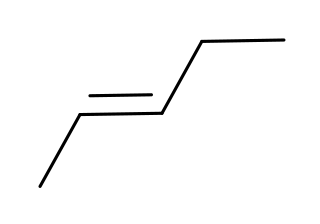
Pentenes are alkenes with the chemical formula C
5H
10. Each contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure, and whether the double bond has a cis or trans form.
The molecular formula C4H8O2 may refer to:
In chemical separation terminology, the raffinate is a product which has had a component or components removed. The product having the removed materials is referred to as the extract. For example, in solvent extraction, the raffinate is the liquid stream which remains after solutes from the original liquid are removed through contact with an immiscible liquid. In metallurgy, raffinating refers to a process in which impurities are removed from liquid material.

The molecular formula C3H6O may refer to:
In chemistry, pentadiene is any hydrocarbon with an open chain of five carbons, connected by two single bonds and two double bonds. All those compounds have the same molecular formula C
5H
8. Specifically, it may be

The molecular formula C5H8 may refer to any of the following hydrocarbons:
The molecular formula C6H12 may refer to following structural isomers:
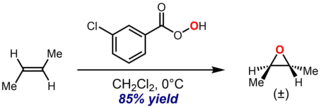
The Prilezhaev reaction, also known as the Prileschajew reaction or Prilezhaev epoxidation, is the chemical reaction of an alkene with a peroxy acid to form epoxides. It is named after Nikolai Prilezhaev, who first reported this reaction in 1909. A widely used peroxy acid for this reaction is meta-chloroperoxybenzoic acid (m-CPBA), due to its stability and good solubility in most organic solvents. An illustrative example is the epoxidation of trans-2-butene with m-CPBA to give trans-2,3-epoxybutane:

2-Methyl-2-butene, 2m2b, 2-methylbut-2-ene, also amylene is an alkene hydrocarbon with the molecular formula C5H10.
The molecular formula C5H10S (molar mass: 102.20 g/mol, exact mass: 102.0503 u) may refer to:
The molecular formula C9H16O2 may refer to:

1,2-Dimethylcyclopropane is a cycloalkane consisting of a cyclopropane ring substituted with two methyl groups attached to adjacent carbon atoms. It has three stereoisomers, one cis-isomer and a pair of trans-enantiomers, which differ depending on the orientation of the two methyl groups. As with other cyclopropanes, ring tension results in a relatively unstable compound.

Cyclohexanedimethanol (CHDM) is a mixture of isomeric organic compounds with formula C6H10(CH2OH)2. It is a colorless low-melting solid used in the production of polyester resins. Commercial samples consist of a mixture of cis and trans isomers. It is a di-substituted derivative of cyclohexane and is classified as a diol, meaning that it has two OH functional groups. Commercial CHDM typically has a cis/trans ratio of 30:70.

(1R,3R)-1,2,3-Trimethylcyclopentane is an organic hydrocarbon alicyclic cycloalkane compound with the molecular formula C8H16. It is a saturated cyclopentane with three methyl substituents branching off carbons 1,2, and 3. The methyl groups off carbons 1 and 3 are trans with respect to each other, while the methyl group off carbon 2 has undefined stereochemistry, allowing it to be either cis or trans with respect to methyl 1 or 3.

4-Methylcyclohexanemethanol (MCHM, systematic name 4-methylcyclohexylmethanol) is an organic compound with the formula CH3C6H10CH2OH. Classified as a saturated higher alicyclic primary alcohol. Both cis and trans isomers exist, depending on the relative positions of the methyl (CH3) and hydroxymethyl (CH2OH) groups on the cyclohexane ring. Commercial samples of MCHM consists of a mixture of these isomers as well as other components that vary with the supplier.

Spiropentane is a hydrocarbon with formula C5H8. It is the simplest spiro-connected cycloalkane, a triangulane. It took several years after the discovery in 1887 until the structure of the molecule was determined. According to the nomenclature rules for spiro compounds, the systematic name is spiro[2.2]pentane. However, there can be no constitutive isomeric spiropentanes, hence the name is unique without brackets and numbers.
Pentenoic acid is any of five mono-carboxylic acids whose molecule has an unbranched chain of five carbons connected by three single bonds and one double bond. That is, any compound with one of the formulas HO(O=)C−CH=CH−CH2−CH3 (2-pentenoic), HO(O=)C−CH2−CH=CH−CH3 (3-pentenoic), or HO(O=)C−CH2−CH2−CH=CH2 (4-pentenoic). In the IUPAC-recommended nomenclature, these acids are called pent-2-enoic, pent-3-enoic, and pent-4-enoic, respectively. All these compounds have the empirical formula C
5H
8O
2.