
In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.
In chemistry, a structural isomer of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomers of alkenes.
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional amount of internal energy which an unstrained molecule does not. This extra internal energy, or strain energy, can be likened to a compressed spring. Much like a compressed spring must be held in place to prevent release of its potential energy, a molecule can be held in an energetically unfavorable conformation by the bonds within that molecule. Without the bonds holding the conformation in place, the strain energy would be released.

In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated.
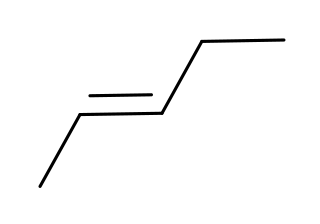
Pentenes are alkenes with the chemical formula C
5H
10. Each molecule contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure and whether the double bond has a cis or trans form.
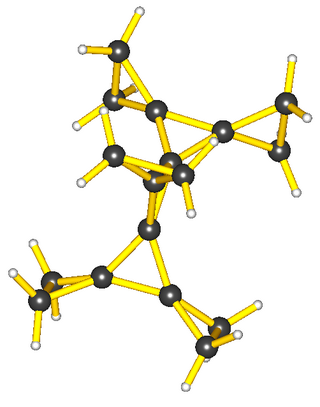
In organic chemistry, spiro compounds are compounds that have at least two molecular rings sharing one common atom. Simple spiro compounds are bicyclic. The presence of only one common atom connecting the two rings distinguishes spiro compounds from other bicyclics. Spiro compounds may be fully carbocyclic or heterocyclic. One common type of spiro compound encountered in educational settings is a heterocyclic one— the acetal formed by reaction of a diol with a cyclic ketone.
In organic chemistry, a ring flip is the interconversion of cyclic conformers that have equivalent ring shapes that results in the exchange of nonequivalent substituent positions. The overall process generally takes place over several steps, involving coupled rotations about several of the molecule's single bonds, in conjunction with minor deformations of bond angles. Most commonly, the term is used to refer to the interconversion of the two chair conformers of cyclohexane derivatives, which is specifically referred to as a chair flip, although other cycloalkanes and inorganic rings undergo similar processes.

In organic chemistry, cyclopropanation refers to any chemical process which generates cyclopropane rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroid insecticides and a number of quinolone antibiotics. However, the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions. Many of the reactions proceed in a cheletropic manner.
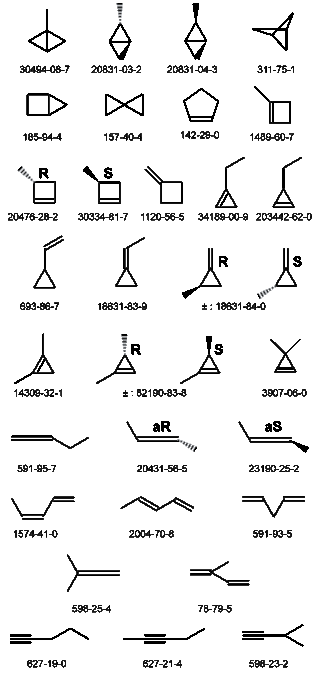
The molecular formula C5H8 may refer to any of the following hydrocarbons:

C5H10 is the molecular formula of 13 hydrocarbon isomers (represented by their CAS numbers on the chart). They can be divided into cycloalkanes and alkenes.
The molecular formula C6H12 may refer to following structural isomers:
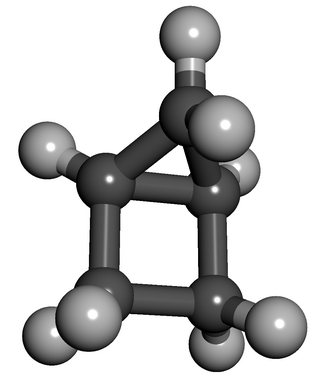
Housane or bicyclo[2.1.0]pentane is a saturated cycloalkane with the formula C5H8. It is a colorless, volatile liquid at room temperature. It was named "housane" because of its shape, which resembles a simple drawing of a house. Structurally, the molecule consists of cyclopropane fused to cyclobutane. The synthesis of molecules containing multiple strained rings, such as housane, is a traditional endeavor in synthetic organic chemistry.

In organic chemistry, a cyclitol is a cycloalkane containing at least three hydroxyl, each attached to a different ring carbon atom. The general formula for an unsubstituted cyclitol is C
nH
2n-x(OH)
x or C
nH
2nO
x where 3 ≤ x ≤ n.

(1R,3R)-1,2,3-Trimethylcyclopentane is an organic hydrocarbon alicyclic cycloalkane compound with the molecular formula C8H16. It is a saturated cyclopentane with three methyl substituents branching off carbons 1,2, and 3. The methyl groups off carbons 1 and 3 are trans with respect to each other, while the methyl group off carbon 2 has undefined stereochemistry, allowing it to be either cis or trans with respect to methyl 1 or 3.

Spiropentane is a hydrocarbon with formula C5H8. It is the simplest spiro-connected cycloalkane, a triangulane. It took several years after the discovery in 1887 until the structure of the molecule was determined. According to the nomenclature rules for spiro compounds, the systematic name is spiro[2.2]pentane. However, there can be no constitutive isomeric spiropentanes, hence the name is unique without brackets and numbers.
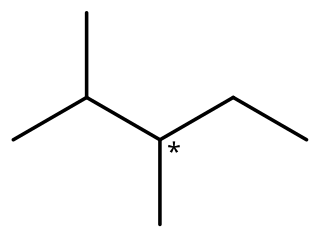
2,3-Dimethylpentane is an organic compound of carbon and hydrogen with formula C
7H
16, more precisely CH
3–CH(CH
3)–CH(CH
3)–CH
2–CH
3: a molecule of pentane with methyl groups –CH
3 replacing hydrogen atoms on carbon atoms 2 and 3. It is an alkane, a fully saturated hydrocarbon; specifically, one of the isomers of heptane.

1,2-Dibromotetrachloroethane (DBTCE) is an organohalide with the chemical formula C2Br2Cl4. It is a crystalline solid that emits lachrymatory (tear-producing) vapours. Dibromotetrachloroethane can be used as a fungicide, flame retardant and a source for bromine in the laboratory. Because the 1,1-dibromotetrachloroethane isomer is rare, 1,2-dibromotetrachloroethane is frequently referred to as simply dibromotetrachloroethane.

















