A methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms — CH3. In formulas, the group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, it can be found on its own in any of three forms: anion, cation or radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction. This dimer can be restored by heating to give the monomer.
A cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon atoms, but has no aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as monomers to produce polymer chains. Due to geometrical considerations, smaller cycloalkenes are almost always the cis isomers, and the term cis tends to be omitted from the names. In larger rings, cis–trans isomerism of the double bond may occur.

Thiourea is an organosulfur compound with the formula SC(NH2)2. It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad class of compounds with the general structure (R1R2N)(R3R4N)C=S. Thioureas are related to thioamides, e.g. RC(S)NR2, where R is methyl, ethyl, etc.
The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act; and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

Cyclopropanation refers to any chemical process which generates cyclopropane rings. It is an important process in modern chemistry as many useful compounds bear this motif; for example pyrethroids and a number of quinolone antibiotics. However the high ring strain present in cyclopropanes makes them challenging to produce and generally requires the use of highly reactive species, such as carbenes, ylids and carbanions. Many of the reactions proceed in a cheletropic manner.
In enzymology, a vitamin-K-epoxide reductase (warfarin-insensitive) is an enzyme that catalyzes the chemical reaction

1-Pentyne, an organic compound, is a terminal alkyne. It is an isomer of 2-pentyne, an internal alkyne.
The Danheiser annulation or Danheiser TMS-cyclopentene annulation is an organic reaction of an α,β-unsaturated ketone and a trialkylsilylallene in the presence of a Lewis Acid to give a trialkylsilylcyclopentene in a regiocontrolled annulation.

2-Pentyne, an organic compound, is an internal alkyne. It is an isomer of 1-pentyne, a terminal alkyne.

Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
In chemistry, pentadienyl refers to the organic radical, anion, or cation with the formula [CH2CHCHCHCH2]z, where z = 0, −1, +1, respectively.

Cyclopentyl methyl ether (CPME), also known as methoxycyclopentane, is hydrophobic ether solvent. A high boiling point of 106 °C (223 °F) and preferable characteristics such as low formation of peroxides, relative stability under acidic and basic conditions, formation of azeotropes with water coupled with a narrow explosion range render CPME an alternative to other ethereal solvents such as tetrahydrofuran (THF), 2-methyltetrahydrofuran (2-MeTHF), dioxane (carcinogenic), and 1,2-dimethoxyethane (DME).
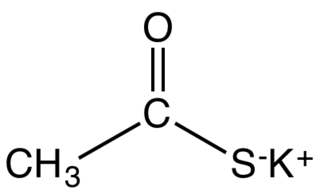
Potassium thioacetate is an organosulfur compound and a salt with the formula CH3COS−K+. This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives.

In organometallic chemistry, the activation of cyclopropanes by transition metals is a research theme with implications for organic synthesis and homogeneous catalysis. Being highly strained, cyclopropanes are prone to oxidative addition to transition metal complexes. The resulting metallacycles are susceptible to a variety of reactions. These reactions are rare examples of C-C bond activation. The rarity of C-C activation processes has been attributed to Steric effects that protect C-C bonds. Furthermore, the directionality of C-C bonds as compared to C-H bonds makes orbital interaction with transition metals less favorable. Thermodynamically, C-C bond activation is more favored than C-H bond activation as the strength of a typical C-C bond is around 90 kcal per mole while the strength of a typical unactivated C-H bond is around 104 kcal per mole.

Rick L. Danheiser is an American organic chemist and is the Arthur C. Cope Professor of Chemistry at the Massachusetts Institute of Technology and Chair of the MIT Faculty. His research involves the invention of new methods for the synthesis of complex organic compounds. Danheiser is known for the Danheiser annulation organic reaction and Danheiser benzannulation chemical reaction.
Vinylcyclopropane [5+2] cycloaddition is a type of cycloaddition between a vinylcyclopropane (VCP) and an olefin or alkyne to form a seven-membered ring.











