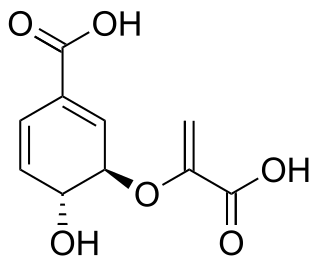
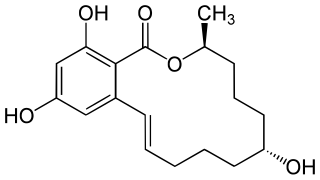
The molecular formula C7H6O4 may refer to:
- Dihydroxybenzoic acids, a type of phenolic acids
- 2,3-Dihydroxybenzoic acid (2-pyrocatechuic acid or hypogallic acid)
- 2,4-Dihydroxybenzoic acid (β-resorcylic acid)
- 2,5-Dihydroxybenzoic acid (gentisic acid)
- 2,6-Dihydroxybenzoic acid (γ-resorcylic acid)
- 3,4-Dihydroxybenzoic acid (protocatechuic acid)
- 3,5-Dihydroxybenzoic acid (α-resorcylic acid)

2,6-Dihydroxybenzoic acid is a dihydroxybenzoic acid. It is a very strong acid due to its intramolecular hydrogen bonding.

3,5-Dihydroxybenzoic acid is a dihydroxybenzoic acid. It is a colorless solid.
- Patulin, a mycotoxin produced by a variety of molds
Dihydroxybenzoic acids (DHBA) are a type of phenolic acids.
2,3-Dihydroxybenzoic acid is a natural phenol found in Phyllanthus acidus and in the aquatic fern Salvinia molesta. It is also abundant in the fruits of Flacourtia inermis. It is a dihydroxybenzoic acid, a type of organic compound. The colorless solid occurs naturally, being formed via the shikimate pathway. It is incorporated into various siderophores, which are molecules that strongly complex iron ions for absorption into bacteria. 2,3-DHB consists of a catechol group, which upon deprotonation binds iron centers very strongly, and the carboxylic acid group by which the ring attaches to various scaffolds through amide bonds. A famous high affinity siderophore is enterochelin, which contains three dihydroxybenzoyl substituents linked to the depsipeptide of serine.
2,4-Dihydroxybenzoic acid is a dihydroxybenzoic acid.
| This set index page lists chemical structure articles associated with the same molecular formula. If an internal link led you here, you may wish to change the link to point directly to the intended article. |