Related Research Articles

Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth.

Henry Joseph Macaulay Barnett, known by his colleagues and friends as "Barney", was a Canadian physician and neurologist. He was also a leading clinical stroke researcher as a result of being the principal investigator in several major clinical trials. As a clinical scientist, he did pioneering research in stroke prevention, beginning with the use of aspirin.

Idebenone is a drug that was initially developed by Takeda Pharmaceutical Company for the treatment of Alzheimer's disease and other cognitive defects. This has been met with limited success. The Swiss company Santhera Pharmaceuticals has started to investigate it for the treatment of neuromuscular diseases. In 2010, early clinical trials for the treatment of Friedreich's ataxia and Duchenne muscular dystrophy have been completed. As of December 2013 the drug is not approved for these indications in North America or Europe. It is approved by the European Medicines Agency (EMA) for use in Leber's hereditary optic neuropathy (LHON) and was designated an orphan drug in 2007.

Desmoteplase is a novel, highly fibrin-specific "clot-busting" (thrombolytic) drug in development that reached phase III clinical trials. The Danish pharmaceutical company, Lundbeck, owns the worldwide rights to Desmoteplase. In 2009, two large trials were started to test it as a safe and effective treatment for patients with acute ischaemic stroke. After disappointing results in DIAS-3, DIAS-4 was terminated, and in December 2014 Lundbeck announced that they would stop the development of desmoteplase.

Agomelatine, sold under the brand names Valdoxan and Thymanax, among others, is an atypical antidepressant most commonly used to treat major depressive disorder and generalized anxiety disorder. One review found that it is as effective as other antidepressants with similar discontinuation rates overall but fewer discontinuations due to side effects. Another review also found it was similarly effective to many other antidepressants.

Oxybutynin, sold under the brand name Ditropan among others, is an anticholinergic medication primarily used to treat overactive bladder. It is widely considered a first-line therapy for overactive bladder due to its well-studied side effect profile, broad applicability, and continued efficacy over long periods of time. It works similar to tolterodine, darifenacin, and solifenacin, although it is usually preferred over these medications. It is sometimes used off-label for treatment of hyperhidrosis, or excessive sweating. It has also been used off-label to treat bed wetting in children, but this use has declined, as it is most likely ineffective in this role. It is taken by mouth or applied to the skin.
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness (efficacy) and safety of medications, devices, diagnostic products, and treatment regimens intended for improving human health. These research procedures are designed for the prevention, treatment, diagnosis or understanding of disease symptoms.

Perindopril is a medication used to treat high blood pressure, heart failure, or stable coronary artery disease.

Latrepirdine is an antihistamine drug which has been used clinically in Russia since 1983.
Recent epidemiologic studies confirm that stroke is the most frequent cause of death in the People's Republic of China, with an incidence more than fivefold that of myocardial infarction. Intracerebral hemorrhage causes about one third of all strokes, nearly three times the frequency in North American stroke registries. A marked regional variation in stroke incidence exists, with a threefold higher stroke incidence in northern than in southern Chinese cities, suggesting important environmental or dietary influences. Stroke treatment often involves a combination of modern and traditional herbal medicine; the latter may modify platelet aggregation and blood viscosity. Stroke, particularly intracerebral hemorrhage, is the most frequent and important vascular disorder in China.
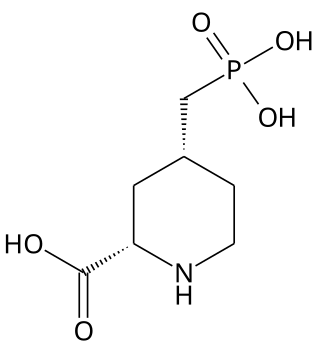
Selfotel (CGS-19755) is a drug which acts as a competitive NMDA antagonist, directly competing with glutamate for binding to the receptor. Initial studies showed it to have anticonvulsant, anxiolytic, analgesic and neuroprotective effects, and it was originally researched for the treatment of stroke, but subsequent animal and human studies showed phencyclidine-like effects, as well as limited efficacy and evidence for possible neurotoxicity under some conditions, and so clinical development was ultimately discontinued.

Terutroban is an antiplatelet agent developed by Servier Laboratories. It is a selective thromboxane prostanoid (TP) antagonist and is an orally active drug in clinical development for the secondary prevention of acute thrombotic complications.

Pomaglumetad (LY-404,039) is an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor group II subtypes mGluR2 and mGluR3. Pharmacological research has focused on its potential antipsychotic and anxiolytic effects. Pomaglumetad is intended as a treatment for schizophrenia and other psychotic and anxiety disorders by modulating glutamatergic activity and reducing presynaptic release of glutamate at synapses in limbic and forebrain areas relevant to these disorders. Human studies investigating therapeutic use of pomaglumetad have focused on the prodrug LY-2140023, a methionine amide of pomaglumetad (also called pomaglumetad methionil) since pomaglumetad exhibits low oral absorption and bioavailability in humans.

Repinotan (BAYx3702), an aminomethylchroman derivative, is a selective 5-HT1A receptor full agonist with high potency and efficacy. It has neuroprotective effects in animal studies, and was trialed in humans for reducing brain injury following head trauma. It was subsequently trialed up to phase II for treatment of stroke, but while side effects were mild and consisted mainly of nausea, repinotan failed to demonstrate sufficient efficacy to justify further clinical trials. However, repinotan continues to be investigated for other applications, and was found to be effective at counteracting the respiratory depression produced by morphine, though with slight reduction in analgesic effects.
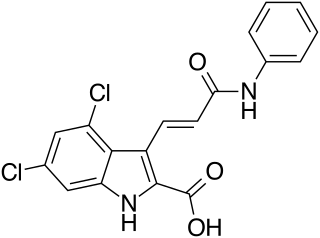
Gavestinel (GV-150,526) was an investigational drug developed by GlaxoSmithKline for acute intracerebral hemorrhage, which in 2001 failed to show an effect in what was at the time, the largest clinical trial in stroke that had been conducted.

The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.

Diffusion Pharmaceuticals Inc (NASDAQ:DFFN) is a publicly traded biotechnology and drug development company based in Charlottesville, Virginia, U.S. It was co-founded in 2001 by American life sciences entrepreneur David Kalergis and University of Virginia Chemical Engineering Professor John L. Gainer. Gainer is the inventor of the company's platform technology of oxygen diffusion-enhancing compounds and its lead drug, trans sodium crocetinate (TSC). TSC acts to increase the rate at which oxygen moves through blood plasma by the process of diffusion, a phenomenon that forms the basis for the company's name. On January 8, 2016, the formerly privately held company merged with Restorgenex Corporation to become a publicly traded NASDAQ-listed company with the trading symbol DFFN. TSC and other oxygen diffusion-enhancing compounds, including bipolar trans carotenoid salts, have been investigated by Diffusion Pharmaceuticals for treatment of conditions associated with reduced oxygen availability in tissues (hypoxia). Most recently, Diffusion has begun the initiation of clinical trials in the U.S. and Eastern Europe for the use of trans sodium crocetinate in the treatment of COVID-19 patients with respiratory distress-related oxygen deficiency and the risk of multiple organ failure.
Jean-Claude Tardif is the Director of the Research Center at the Montreal Heart Institute and Professor of Medicine at the University of Montreal. He received his medical degree (MD) in 1987 from the University of Montreal and specialized in cardiology and research in Montreal and Boston until 1994. Dr. Tardif holds the Canada Research Chair in personalized medicine and the University of Montreal endowed research chair in atherosclerosis. He is also the Scientific Director of the Montreal Health Innovations Coordinating Center (MHICC).
A cerebroprotectant is a drug that is intended to protect the brain after the onset of acute ischemic stroke. As stroke is the second largest cause of death worldwide and a leading cause of adult disability, over 150 drugs have been tested in clinical trials to provide cerebroprotection.
References
- ↑ This trial is currently recruiting patients and details can be found at the ClinicalTrials.gov database from NIH under reference NCT00554723.