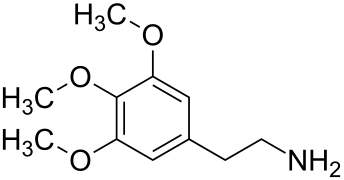
Mescaline or mescalin (3,4,5-trimethoxyphenethylamine) is a naturally occurring psychedelic protoalkaloid of the substituted phenethylamine class, known for its hallucinogenic effects comparable to those of LSD and psilocybin.
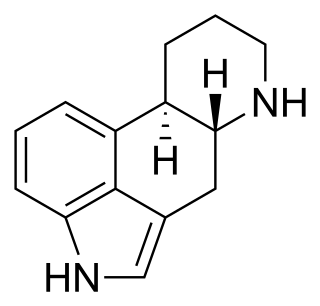
Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-catalyzed processes where chemical substances absorbed as nutrients serve as enzyme substrates, with conversion by the living organism either into simpler or more complex products. Examples of biosynthetic pathways include those for the production of amino acids, lipid membrane components, and nucleotides, but also for the production of all classes of biological macromolecules, and of acetyl-coenzyme A, adenosine triphosphate, nicotinamide adenine dinucleotide and other key intermediate and transactional molecules needed for metabolism. Thus, in biosynthesis, any of an array of compounds, from simple to complex, are converted into other compounds, and so it includes both the catabolism and anabolism of complex molecules. Biosynthetic processes are often represented via charts of metabolic pathways. A particular biosynthetic pathway may be located within a single cellular organelle, while others involve enzymes that are located across an array of cellular organelles and structures.

Lophophine is a putative psychedelic and entactogen drug of the methylenedioxyphenethylamine class. It is the α-demethylated homologue of MMDA, and is also closely related to mescaline.

Dermatophyllum secundiflorum is a species of flowering shrub or small tree in the family Fabaceae that is native to the Southwestern United States and Mexico. Its common names include Texas mountain laurel, Texas mescalbean, frijolito, and frijolillo.

3,4-Dimethoxyphenethylamine (DMPEA) is a chemical compound of the phenethylamine class. It is an analogue of the major human neurotransmitter dopamine where the 3- and 4-position hydroxy groups have been replaced with methoxy groups. It is also closely related to mescaline which is 3,4,5-trimethoxyphenethylamine.

Lophophora diffusa, commonly known as false peyote, is a species of plant in the family Cactaceae and one of the species in the Lophophora genus. It is endemic to Mexico in the outskirts of Querétaro. This species contains zero to trace amounts of mescaline; pellotine, whose psychoactive effects are comparatively minimal, is the principal alkaloid. The species name diffusa refers to the flat tubercles that are outspread without the plant having prominent ribs.

Deoxyepinephrine, also known by the common names N-methyldopamine and epinine, is an organic compound and natural product that is structurally related to the important neurotransmitters dopamine and epinephrine. All three of these compounds also belong to the catecholamine family. The pharmacology of epinine largely resembles that of its "parent", dopamine. Epinine has been found in plants, insects and animals. It is also of significance as the active metabolic breakdown product of the prodrug ibopamine, which has been used to treat congestive heart failure.

Trichocereus macrogonus var. pachanoi is a fast-growing columnar cactus found in the Andes at 2,000–3,000 m (6,600–9,800 ft) in altitude. It is one of a number of kinds of cacti known as San Pedro cactus. It is native to Ecuador, Peru and Colombia, but also found in Argentina, Bolivia, Chile and Venezuela and cultivated in other parts of the world. Uses for it include traditional medicine and traditional veterinary medicine, and it is widely grown as an ornamental cactus. It has been used for healing and religious divination in the Andes Mountains region for over 3,000 years.

Lophophora is a genus of spineless, button-like cacti. Its native range covers Texas through Mexico to southwestern Mexico. The species are extremely slow growing, sometimes taking up to thirty years to reach flowering age in the wild. Cultivated specimens grow considerably faster, usually taking between three and ten years to reach from seedling to mature flowering adult. The slow rate of reproduction and over-harvesting by collectors render the species under threat in the wild.

The peyote is a small, spineless cactus which contains psychoactive alkaloids, particularly mescaline. Peyote is a Spanish word derived from the Nahuatl peyōtl, meaning "caterpillar cocoon", from a root peyōni, "to glisten". Peyote is native to Mexico and southwestern Texas. It is found primarily in the Sierra Madre Occidental, the Chihuahuan Desert and in the states of Nayarit, Coahuila, Nuevo León, Tamaulipas, and San Luis Potosí among scrub. It flowers from March to May, and sometimes as late as September. The flowers are pink, with thigmotactic anthers.
Many cacti are known to be psychoactive, containing phenethylamine alkaloids such as mescaline. However, the two main ritualistic (folkloric) genera are Echinopsis, of which the most psychoactive species occur in the San Pedro cactus group, and Lophophora, with peyote being the most psychoactive species. Several other species pertaining to other genera are also psychoactive, though not always used with a ritualistic intent.
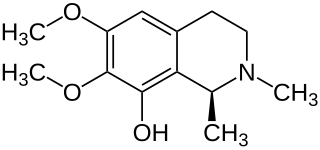
Pellotine is an alkaloid found in Lophophora species, in particular L. diffusa. Pellotine is slightly narcotic, and has been used by Native Americans as a constituent of peyote for sacramental purposes.

Anhalonidine a naturally occurring alkaloid which can be isolated from certain members of the cactus family, such as Lophophora. It is structurally related to mescaline.
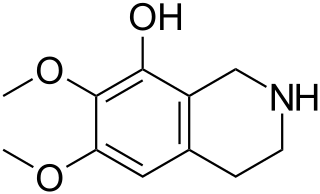
Anhalamine is a naturally occurring alkaloid which can be isolated from Lophophora williamsii. It is structurally related to mescaline.

Anhalidine is a naturally occurring tetrahydroisoquinoline based alkaloid which can be isolated from Lophophora williamsii; it has also been detected other cactii and several species of Acacia. It is part of a family of compounds that are structurally related to mescaline.
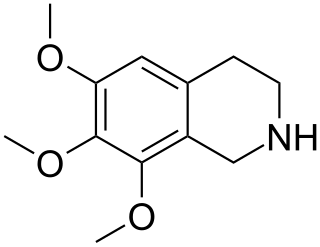
Anhalinine is a naturally occurring alkaloid which can be isolated from Lophophora williamsii. It is structurally related to mescaline.

Psychoactive plants are plants, or preparations thereof, that upon ingestion induce psychotropic effects. As stated in a reference work:
Psychoactive plants are plants that people ingest in the form of simple or complex preparations in order to affect the mind or alter the state of consciousness.
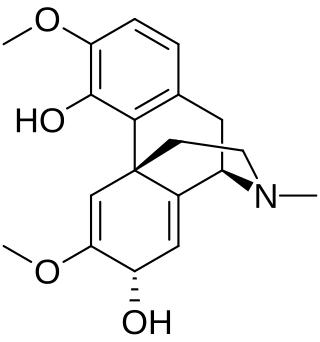
Salutaridinol is a modified benzyltetrahydroisoquinoline alkaloid with the formula C19H23NO4. It is produced in the secondary metabolism of the opium poppy Papaver somniferum (Papaveraceae) as an intermediate in the biosynthetic pathway that generates morphine. As an isoquinoline alkaloid, it is fundamentally derived from tyrosine as part of the shikimate pathway of secondary metabolism. Salutaridinol is a product of the enzyme salutaridine: NADPH 7-oxidoreductase and the substrate for the enzyme salutaridinol 7-O-acetyltransferase, which are two of the four enzymes in the morphine biosynthesis pathway that generates morphine from (R)-reticuline. Salutaridinol's unique position adjacent to two of the four enzymes in the morphine biosynthesis pathway gives it an important role in enzymatic, genetic, and synthetic biology studies of morphine biosynthesis. Salutaridinol levels are indicative of the flux through the morphine biosynthesis pathway and the efficacy of both salutaridine: NADPH 7-oxidoreductase and salutaridinol 7-O-acetyltransferase.
Erythrina alkaloids, generally containing benzyl-tetrahydroisoquinoline structure, are widely distributed in Erythrina species, a genus of plants which belong to the Fabaceae family in tropical and subtropical regions. The Erythrina alkaloids can be found in several organs of Erythrina trees but are primarily found in their seeds. They display several unique properties, and are the subject of active scientific research relating to their synthesis and bioactivity.