Lonicera japonica, known as Japanese honeysuckle and golden-and-silver honeysuckle, is a species of honeysuckle native to eastern Asia including China, Japan, and Korea. It is a twining vine able to climb up to 10 m (33 ft) high or more in trees, with opposite, simple oval leaves 3–8 cm (1.2–3.1 in) long and 2–3 cm (0.79–1.18 in) broad. The flowers are double-tongued, opening white and fading to yellow, and sweetly vanilla scented. The fruit is a black spherical berry 3–4 mm (0.12–0.16 in) diameter containing a few seeds.

A coffee bean is a seed of the coffee plant and the source for coffee. It is the pit inside the red or purple fruit often referred to as a cherry. Just like ordinary cherries, the coffee fruit is also a so-called stone fruit. Even though the coffee beans are seeds, they are referred to as "beans" because of their resemblance to true beans. The fruits – coffee cherries or coffee berries – most commonly contain two stones with their flat sides together. A small percentage of cherries contain a single seed, instead of the usual two. This is called a "peaberry". The peaberry occurs only between 10 and 15% of the time, and it is a fairly common belief that they have more flavour than normal coffee beans. Like Brazil nuts and white rice, coffee beans consist mostly of endosperm.

Gingerol, properly as [6]-gingerol, is a chemical compound found in fresh ginger. Chemically, gingerol is a relative of capsaicin and piperine, the compounds which give chilli peppers and black pepper their respective spiciness. It is normally found as a pungent yellow oil, but also can form a low-melting crystalline solid.

Cyanidin is a natural organic compound. It is a particular type of anthocyanidin. It is a pigment found in many red berries including grapes, bilberry, blackberry, blueberry, cherry, cranberry, elderberry, hawthorn, loganberry, açai berry and raspberry. It can also be found in other fruits such as apples and plums, and in red cabbage and red onion. It has a characteristic reddish-purple color, though this can change with pH; solutions of the compound are red at pH < 3, violet at pH 7-8, and blue at pH > 11. In certain fruits, the highest concentrations of cyanidin are found in the seeds and skin. In a recent study, cyanidin was found to be a potent sirtuin 6 (SIRT6) activator.
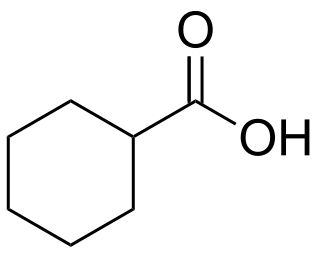
Cyclohexanecarboxylic acid is the organic compound with the formula C6H11CO2H. It is the carboxylic acid of cyclohexane. It is an oily colorless oil that crystallizes near room temperature.
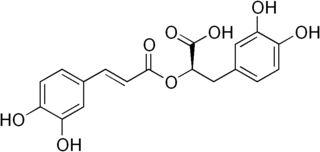
Rosmarinic acid is a chemical compound found in a variety of plants.
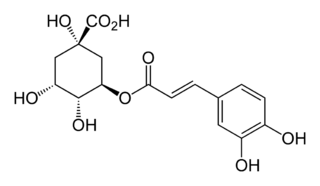
In enzymology, a 5-O-(4-coumaroyl)-D-quinate 3'-monooxygenase (EC 1.14.13.36) is an enzyme that catalyzes the chemical reaction

Artemisia princeps, also called ssuk, Korean wormwood, Korean mugwort, and Japanese mugwort in English, is an Asian plant species in the sunflower family, native to China, Japan, and Korea. It is a perennial, very vigorous plant that grows to 1.2 meters. This species spreads rapidly by means of underground stolons and can become invasive. It bears small, buff-colored flowers from July to November which are hermaphroditic, and pollinated by wind. The leaves are feather shaped, scalloped and light green, with white dense fuzz on the underside.
The molecular formula C16H18O9 may refer to:

Phyllostachys edulis, moso bamboo, or tortoise-shell bamboo, or mao zhu, is a temperate species of giant timber bamboo native to China and Taiwan and naturalised elsewhere, including Japan where it is widely distributed south of Hokkaido. The edulis part of the Latin name refers to its edible shoots. This bamboo can reach heights of up to 28 m (92 ft). This particular species of bamboo is the most common species used in the bamboo textile industry of China, for the production of rayon.

Scorzoneroides or hawkbits is a genus of plants of dandelion tribe within the daisy family.

Pteris ensiformis, the slender brake, silver lace fern, sword brake fern, or slender brake fern, is a plant species in the genus Pteridoideae subfamily of the Pteridaceae. It is found in Asia and the Pacific.

Neochlorogenic acid is a natural polyphenolic compound found in some types of dried fruits and a variety of other plant sources such as peaches. It is an isomer of chlorogenic acid; both of these are members of the caffeoylquinic acid class of molecules.
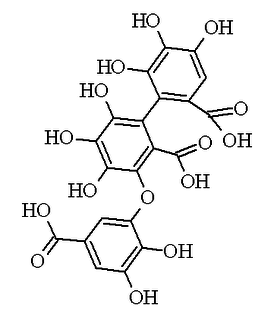
Sanguisorbic acid is a constituent of some ellagitannins. It is constituted by a hexahydroxydiphenic acid unit linked by an O-C bond to a gallic acid. The differences with its isomers, valoneic acid and nonahydroxytriphenic acid, are that the hydroxyl that links the hexahydroxydiphenoyl (HHDP) group to the galloyl group belongs to the galloyl group in valoneic acid, while in nonahydroxytriphenic acid, the hexahydroxydiphenic acid unit is linked by a C-C bond to gallic acid.

4-Caffeoyl-1,5-quinide is found in roasted coffee beans. It is formed by lactonization of 4-O-caffeoylquinic acid during the roasting process.