
The sodium–potassium pump is an enzyme found in the membrane of all animal cells. It performs several functions in cell physiology.

The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.
SERCA, or sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, or SR Ca2+-ATPase, is a calcium ATPase-type P-ATPase. Its major function is to transport calcium from the cytosol into the sarcoplasmic reticulum.

Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.
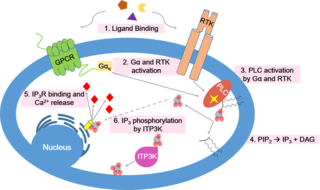
Calcium signaling is the use of calcium ions (Ca2+) to communicate and drive intracellular processes often as a step in signal transduction. Ca2+ is important for cellular signalling, for once it enters the cytosol of the cytoplasm it exerts allosteric regulatory effects on many enzymes and proteins. Ca2+ can act in signal transduction resulting from activation of ion channels or as a second messenger caused by indirect signal transduction pathways such as G protein-coupled receptors.

Phospholamban, also known as PLN or PLB, is a micropeptide protein that in humans is encoded by the PLN gene. Phospholamban is a 52-amino acid integral membrane protein that regulates the calcium (Ca2+) pump in cardiac muscle cells.

T-tubules are extensions of the cell membrane that penetrate into the center of skeletal and cardiac muscle cells. With membranes that contain large concentrations of ion channels, transporters, and pumps, T-tubules permit rapid transmission of the action potential into the cell, and also play an important role in regulating cellular calcium concentration.
Calcium-induced calcium release (CICR) describes a biological process whereby calcium is able to activate calcium release from intracellular Ca2+ stores (e.g., endoplasmic reticulum or sarcoplasmic reticulum). Although CICR was first proposed for skeletal muscle in the 1970s, it is now known that CICR is unlikely to be the primary mechanism for activating SR calcium release. Instead, CICR is thought to be crucial for excitation-contraction coupling in cardiac muscle. It is now obvious that CICR is a widely occurring cellular signaling process present even in many non-muscle cells, such as in the insulin-secreting pancreatic beta cells, epithelium, and many other cells. Since CICR is a positive-feedback system, it has been of great interest to elucidate the mechanism(s) responsible for its termination.
The sodium-calcium exchanger (often denoted Na+/Ca2+ exchanger, exchange protein, or NCX) is an antiporter membrane protein that removes calcium from cells. It uses the energy that is stored in the electrochemical gradient of sodium (Na+) by allowing Na+ to flow down its gradient across the plasma membrane in exchange for the countertransport of calcium ions (Ca2+). A single calcium ion is exported for the import of three sodium ions. The exchanger exists in many different cell types and animal species. The NCX is considered one of the most important cellular mechanisms for removing Ca2+.

Ca2+ ATPase is a form of P-ATPase that transfers calcium after a muscle has contracted. The two kinds of calcium ATPase are:

The plasma membrane Ca2+ ATPase (PMCA) is a transport protein in the plasma membrane of cells that functions as a calcium pump to remove calcium (Ca2+) from the cell. PMCA function is vital for regulating the amount of Ca2+ within all eukaryotic cells. There is a very large transmembrane electrochemical gradient of Ca2+ driving the entry of the ion into cells, yet it is very important that they maintain low concentrations of Ca2+ for proper cell signalling. Thus, it is necessary for cells to employ ion pumps to remove the Ca2+. The PMCA and the sodium calcium exchanger (NCX) are together the main regulators of intracellular Ca2+ concentrations. Since it transports Ca2+ into the extracellular space, the PMCA is also an important regulator of the calcium concentration in the extracellular space.
A calcium spark is the microscopic release of calcium (Ca2+) from a store known as the sarcoplasmic reticulum (SR), located within muscle cells. This release occurs through an ion channel within the membrane of the SR, known as a ryanodine receptor (RyR), which opens upon activation. This process is important as it helps to maintain Ca2+ concentration within the cell. It also initiates muscle contraction in skeletal and cardiac muscles and muscle relaxation in smooth muscles. Ca2+ sparks are important in physiology as they show how Ca2+ can be used at a subcellular level, to signal both local changes, known as local control, as well as whole cell changes.
ATP2A2 also known as sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) is an ATPase associated with Darier's disease and Acrokeratosis verruciformis.
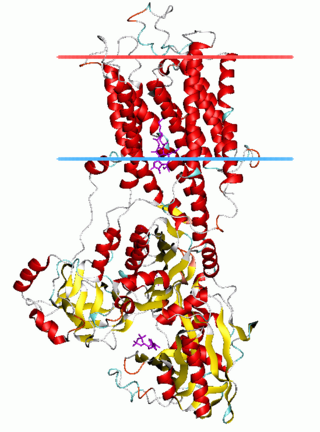
The P-type ATPases, also known as E1-E2 ATPases, are a large group of evolutionarily related ion and lipid pumps that are found in bacteria, archaea, and eukaryotes. P-type ATPases are α-helical bundle primary transporters named based upon their ability to catalyze auto- (or self-) phosphorylation (hence P) of a key conserved aspartate residue within the pump and their energy source, adenosine triphosphate (ATP). In addition, they all appear to interconvert between at least two different conformations, denoted by E1 and E2. P-type ATPases fall under the P-type ATPase (P-ATPase) Superfamily (TC# 3.A.3) which, as of early 2016, includes 20 different protein families.
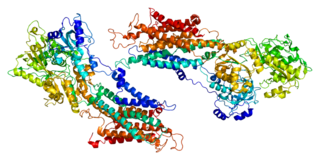
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 (SERCA1) also known as Calcium pump 1, is an enzyme that in humans is encoded by the ATP2A1 gene.

Sarcoplasmic/endoplasmic reticulum calcium ATPase 3 is an enzyme that in humans is encoded by the ATP2A3 gene.

Triadin, also known as TRDN, is a human gene associated with the release of calcium ions from the sarcoplasmic reticulum triggering muscular contraction through calcium-induced calcium release. Triadin is a multiprotein family, arising from different processing of the TRDN gene on chromosome 6. It is a transmembrane protein on the sarcoplasmic reticulum due to a well defined hydrophobic section and it forms a quaternary complex with the cardiac ryanodine receptor (RYR2), calsequestrin (CASQ2) and junctin proteins. The luminal (inner compartment of the sarcoplasmic reticulum) section of Triadin has areas of highly charged amino acid residues that act as luminal Ca2+ receptors. Triadin is also able to sense luminal Ca2+ concentrations by mediating interactions between RYR2 and CASQ2. Triadin has several different forms; Trisk 95 and Trisk 51, which are expressed in skeletal muscle, and Trisk 32 (CT1), which is mainly expressed in cardiac muscle.

Sarcolipin is a micropeptide protein that in humans is encoded by the SLN gene.
Chikashi Toyoshima (Japanese: 豊島 近, Hepburn: Toyoshima Chikashi; born July 17, 1954) is a Japanese biophysicist, a professor at the University of Tokyo and a Foreign Associate of the National Academy of Sciences in the United States. His research focuses on two proteins: the Ca2+ ATPase, and the Na+/ K+-ATPase. Toyoshima's research about Ca2+ ATPase started in 1989. He and his colleagues obtained the world's first series of images of Ca2+ ATPase at the atomic level. He has determined the crystal structures of ten intermediates of Ca2+ ATPase via X-ray crystallography, cryogenic electron microscopy (cryo-EM), among other methods. Toyoshima and Poul Nissen were awarded the Gregori Aminoff Prize in 2016 by The Royal Swedish Academy of Sciences for their fundamental contributions to understanding the structural basis for ATP-driven translocation of ions across membranes.












