
Medical cannabis, or medical marijuana (MMJ), is cannabis and cannabinoids that are prescribed by physicians for their patients. The use of cannabis as medicine has not been rigorously tested due to production and governmental restrictions, resulting in limited clinical research to define the safety and efficacy of using cannabis to treat diseases.

Gilead Sciences, Inc. is an American biopharmaceutical company headquartered in Foster City, California that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and COVID-19, including ledipasvir/sofosbuvir and sofosbuvir. Gilead is a member of the NASDAQ Biotechnology Index and the S&P 500.

Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. As of 2022, clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that cannabidiol is effective for these conditions. CBD is also sold as a herbal dietary supplement promoted with unproven claims of particular therapeutic effects.
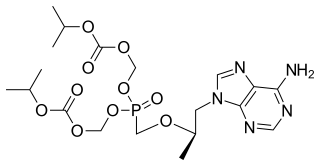
Tenofovir disoproxil, sold under the trade name Viread among others, is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention of HIV/AIDS among those at high risk before exposure, and after a needlestick injury or other potential exposure. It is sold both by itself and together in combinations such as emtricitabine/tenofovir, efavirenz/emtricitabine/tenofovir, and elvitegravir/cobicistat/emtricitabine/tenofovir. It does not cure HIV/AIDS or hepatitis B. It is available by mouth as a tablet or powder.
The Multidisciplinary Association for Psychedelic Studies (MAPS) is an American nonprofit organization working to raise awareness and understanding of psychedelic substances. MAPS was founded in 1986 by Rick Doblin and is now based in San Jose, California.

In the United States, the removal of cannabis from Schedule I of the Controlled Substances Act is a proposed legal and administrative change in cannabis-related law at the federal level. It has been proposed repeatedly since 1972. The category is the most tightly restricted category reserved for drugs that have "no currently accepted medical use."

The National Institute on Drug Abuse (NIDA) is a United States federal government research institute whose mission is to "advance science on the causes and consequences of drug use and addiction and to apply that knowledge to improve individual and public health."

Efavirenz/emtricitabine/tenofovir, sold under the brand name Atripla among others, is a fixed-dose combination antiretroviral medication used to treat HIV/AIDS. It contains efavirenz, emtricitabine, and tenofovir disoproxil. It can be used by itself or together with other antiretroviral medications. It is taken by mouth.
Expanded access or compassionate use is the use of an unapproved drug or medical device under special forms of investigational new drug applications (IND) or IDE application for devices, outside of a clinical trial, by people with serious or life-threatening conditions who do not meet the enrollment criteria for the clinical trial in progress.
Numerous governmental and non-governmental organizations have criticized the U. S. Food and Drug Administration for alleged excessive and/or insufficient regulation. The U.S. Food and Drug Administration (FDA) is an agency of the United States Department of Health and Human Services and is responsible for the safety regulation of most types of foods, dietary supplements, drugs, vaccines, biological medical products, blood products, medical devices, radiation-emitting devices, veterinary products, and cosmetics. The FDA also enforces section 361 of the Public Health Service Act and the associated regulations, including sanitation requirements on interstate travel as well as specific rules for control of disease on products ranging from animals sold as pets to donations of human blood and tissue.
Geoffrey William Guy is a British pharmacologist, physician, businessman and academic, who co-founded GW Pharmaceuticals and has developed treatments using compounds found in cannabis, which are the first cannabis-based medicines approved by and available on the British National Health Service (NHS).

Margaret Ann "Peggy" Hamburg is an American physician and public health administrator, who is serving as the chair of the board of the American Association for the Advancement of Science (AAAS) and co-chair of the InterAcademy Partnership (IAP). She served as the 21st Commissioner of the U.S. Food and Drug Administration from May 2009 to April 2015.

Dronabinol (INN), also known under the trade names Marinol and Syndros, is a generic name for the molecule of delta-9-tetrahydrocannabinol in the pharmaceutical context. It has indications as an appetite stimulant, antiemetic, and sleep apnea reliever and is approved by the FDA as safe and effective for HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting only.
Igor Grant is an American psychiatrist. He is Distinguished Professor in the Department of Psychiatry in the School of Medicine at the University of California, San Diego. He is Director of the HIV Neurobehavioral Research Program (HNRP) and the Center for Medicinal Cannabis Research (CMCR). Grant is the founding Editor of the Journal of the International Neuropsychological Society and founding co-editor of the journal AIDS and Behavior. His work focuses on effects of HIV and drug use, particularly alcohol, medical marijuana, and methamphetamine.
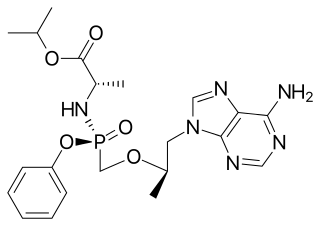
Tenofovir alafenamide, sold under the brand name Vemlidy, is an antiviral medication used against hepatitis B and HIV. It is used for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease and is given in combination with other medications for the prevention and treatment of HIV. It is taken by mouth.

GW Pharmaceuticals Limited is a British pharmaceutics company known for its multiple sclerosis treatment product nabiximols which was the first natural cannabis plant derivative to gain market approval in any country. Another cannabis-based product, Epidiolex, was approved for treatment of epilepsy by the US Food and Drug Administration in 2018. It is a subsidiary of Jazz Pharmaceuticals.

Scott Gottlieb is an American physician, investor, and author who served as the 23rd commissioner of the Food and Drug Administration (FDA) from May 2017 until April 2019. He is presently a senior fellow at the conservative think tank the American Enterprise Institute (AEI), a partner at the venture capital firm New Enterprise Associates (NEA), a member of the board of directors of drug maker Pfizer, Inc and gene sequencing company Illumina, Inc., and a contributor to the cable financial news network CNBC and the CBS News program Face the Nation. An elected member of the National Academy of Medicine, Gottlieb is the author of The New York Times best selling book Uncontrolled Spread on the COVID-19 pandemic and the national security vulnerabilities that it revealed. His forthcoming book, The Miracle Century: Making Sense of the Cell Therapy Revolution, traces the scientific achievements that propelled progress in cell therapies.
Young blood transfusion refers to transfusing blood specifically from a young person into an older one with the intention of creating a health benefit. The scientific community currently views the practice as essentially pseudoscientific, with comparisons to snake oil. There are also concerns of harm. The U.S. Food and Drug Administration, in 2019, cautioned "consumers against receiving young donor plasma infusions" stating that they are an "unproven treatment".
Viatris Inc. is an American global pharmaceutical and healthcare corporation headquartered in Canonsburg, Pennsylvania. The corporation was formed through the merger of Mylan and Upjohn, a legacy division of Pfizer, on November 16, 2020.

John R. Mascola is an American physician-scientist, immunologist and infectious disease specialist. He was the director of the Vaccine Research Center (VRC), part of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). He also served as a principal advisor to Anthony Fauci, director of NIAID, on vaccines and biomedical research affairs. Mascola is the current Chief Scientific Officer for ModeX Therapeutics.










