
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), and is typically associated with the corrosion of refined iron.

Stainless steel, also known as inox or Corrosion Resistant (Stainless) Steel (CRES), is an alloy of iron that is resistant to rusting and corrosion. It contains at least 10.5% chromium, usually nickel, and may also contain other elements, such as carbon, to obtain the desired properties. Stainless steel's resistance to corrosion results from the chromium, which forms a passive film that can protect the material and self-heal in the presence of oxygen.

Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply.

Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.

A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged metal structures from corrosion.
Cupronickel or copper-nickel (CuNi) is an alloy of copper that contains nickel and strengthening elements, such as iron and manganese. The copper content typically varies from 60 to 90 percent.

Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects the metal to be protected to a more easily corroded "sacrificial metal" to act as the anode. The sacrificial metal then corrodes instead of the protected metal. For structures such as long pipelines, where passive galvanic cathodic protection is not adequate, an external DC electrical power source is used to provide sufficient current.
Aluminium–silicon alloys or Silumin is a general name for a group of lightweight, high-strength aluminium alloys based on an aluminum–silicon system (AlSi) that consist predominantly of aluminum - with silicon as the quantitatively most important alloying element. Pure AlSi alloys cannot be hardened, the commonly used alloys AlSiCu and AlSiMg can be hardened. The hardening mechanism corresponds to that of AlCu and AlMgSi. The rarely used wrought alloys in the 4000 series and the predominantly used cast alloys are standardised in the 40000 series.

Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.
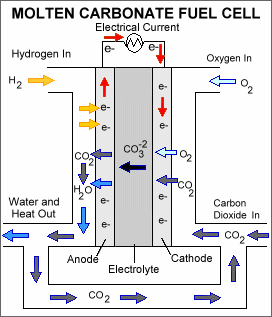
Molten-carbonate fuel cells (MCFCs) are high-temperature fuel cells that operate at temperatures of 600 °C and above.

Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can permeate solid metals. Once absorbed, hydrogen lowers the stress required for cracks in the metal to initiate and propagate, resulting in embrittlement. Hydrogen embrittlement occurs most notably in steels, as well as in iron, nickel, titanium, cobalt, and their alloys. Copper, aluminium, and stainless steels are less susceptible to hydrogen embrittlement.

Pitting corrosion, or pitting, is a form of extremely localized corrosion that leads to the random creation of small holes in metal. The driving power for pitting corrosion is the depassivation of a small area, which becomes anodic while an unknown but potentially vast area becomes cathodic, leading to very localized galvanic corrosion. The corrosion penetrates the mass of the metal, with a limited diffusion of ions.

Alclad is a corrosion-resistant aluminium sheet formed from high-purity aluminium surface layers metallurgically bonded to high-strength aluminium alloy core material. It has a melting point of about 500 °C (932 °F). Alclad is a trademark of Alcoa but the term is also used generically.
Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. The process involves electroplating, running a current of electricity through a saline/zinc solution with a zinc anode and steel conductor. Such Zinc electroplating or Zinc alloy electroplating maintains a dominant position among other electroplating process options, based upon electroplated tonnage per annum. According to the International Zinc Association, more than 5 million tons are used yearly for both hot dip galvanizing and electroplating. The plating of zinc was developed at the beginning of the 20th century. At that time, the electrolyte was cyanide based. A significant innovation occurred in the 1960s, with the introduction of the first acid chloride based electrolyte. The 1980s saw a return to alkaline electrolytes, only this time, without the use of cyanide. The most commonly used electrogalvanized cold rolled steel is SECC, acronym of "Steel, Electrogalvanized, Cold-rolled, Commercial quality". Compared to hot dip galvanizing, electroplated zinc offers these significant advantages:
Corrosion engineering is an engineering specialty that applies scientific, technical, engineering skills, and knowledge of natural laws and physical resources to design and implement materials, structures, devices, systems, and procedures to manage corrosion. From a holistic perspective, corrosion is the phenomenon of metals returning to the state they are found in nature. The driving force that causes metals to corrode is a consequence of their temporary existence in metallic form. To produce metals starting from naturally occurring minerals and ores, it is necessary to provide a certain amount of energy, e.g. Iron ore in a blast furnace. It is therefore thermodynamically inevitable that these metals when exposed to various environments would revert to their state found in nature. Corrosion and corrosion engineering thus involves a study of chemical kinetics, thermodynamics, electrochemistry and materials science.

Copper alloys are important netting materials in aquaculture. Various other materials including nylon, polyester, polypropylene, polyethylene, plastic-coated welded wire, rubber, patented twine products, and galvanized steel are also used for netting in aquaculture fish enclosures around the world. All of these materials are selected for a variety of reasons, including design feasibility, material strength, cost, and corrosion resistance.
Nickel electroplating is a technique of electroplating a thin layer of nickel onto a metal object. The nickel layer can be decorative, provide corrosion resistance, wear resistance, or used to build up worn or undersized parts for salvage purposes.
The Zinagizado is an electrochemical process to provide a ferrous metal material with anti-corrosive properties. It involves the application of a constant electric current through a circuit to break the bonds and these are attached to the metal to be coated by forming a surface coating. The alloy used is called Zinag (Zn-Al-Ag); this alloy has excellent mechanical and corrosive properties, so the piece will have increased by 60% of life.

Titanium was first introduced into surgeries in the 1950s after having been used in dentistry for a decade prior. It is now the metal of choice for prosthetics, internal fixation, inner body devices, and instrumentation. Titanium is used from head to toe in biomedical implants. One can find titanium in neurosurgery, bone conduction hearing aids, false eye implants, spinal fusion cages, pacemakers, toe implants, and shoulder/elbow/hip/knee replacements along with many more. The main reason why titanium is often used in the body is due to titanium's biocompatibility and, with surface modifications, bioactive surface. The surface characteristics that affect biocompatibility are surface texture, steric hindrance, binding sites, and hydrophobicity (wetting). These characteristics are optimized to create an ideal cellular response. Some medical implants, as well as parts of surgical instruments are coated with titanium nitride (TiN).
1. Potgieter J. H., Heyns A.M., Skinner W. 1990. Cathodic modification as a means of improving the corrosion resistance of alloys. Journal of Applied Electrochemistry, 20: 711 - 715.












