
Studies that are in vivo are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and plants, as opposed to a tissue extract or dead organism. This is not to be confused with experiments done in vitro, i.e., in a laboratory environment using test tubes, Petri dishes, etc. Examples of investigations in vivo include: the pathogenesis of disease by comparing the effects of bacterial infection with the effects of purified bacterial toxins; the development of non-antibiotics, antiviral drugs, and new drugs generally; and new surgical procedures. Consequently, animal testing and clinical trials are major elements of in vivo research. In vivo testing is often employed over in vitro because it is better suited for observing the overall effects of an experiment on a living subject. In drug discovery, for example, verification of efficacy in vivo is crucial, because in vitro assays can sometimes yield misleading results with drug candidate molecules that are irrelevant in vivo.

Proteomics is the large-scale study of proteins. Proteins are vital macromolecules of all living organisms, with many functions such as the formation of structural fibers of muscle tissue, enzymatic digestion of food, or synthesis and replication of DNA. In addition, other kinds of proteins include antibodies that protect an organism from infection, and hormones that send important signals throughout the body.

Plasmodium is a genus of unicellular eukaryotes that are obligate parasites of vertebrates and insects. The life cycles of Plasmodium species involve development in a blood-feeding insect host which then injects parasites into a vertebrate host during a blood meal. Parasites grow within a vertebrate body tissue before entering the bloodstream to infect red blood cells. The ensuing destruction of host red blood cells can result in malaria. During this infection, some parasites are picked up by a blood-feeding insect, continuing the life cycle.
In vitro toxicity testing is the scientific analysis of the toxic effects of chemical substances on cultured bacteria or mammalian cells. In vitro testing methods are employed primarily to identify potentially hazardous chemicals and/or to confirm the lack of certain toxic properties in the early stages of the development of potentially useful new substances such as therapeutic drugs, agricultural chemicals and food additives.
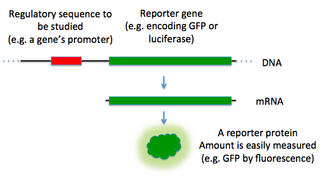
In molecular biology, a reporter gene is a gene that researchers attach to a regulatory sequence of another gene of interest in bacteria, cell culture, animals or plants. Such genes are called reporters because the characteristics they confer on organisms expressing them are easily identified and measured, or because they are selectable markers. Reporter genes are often used as an indication of whether a certain gene has been taken up by or expressed in the cell or organism population.

Plate readers, also known as microplate readers or microplate photometers, are instruments which are used to detect biological, chemical or physical events of samples in microtiter plates. They are widely used in research, drug discovery, bioassay validation, quality control and manufacturing processes in the pharmaceutical and biotechnological industry and academic organizations. Sample reactions can be assayed in 1-1536 well format microtiter plates. The most common microplate format used in academic research laboratories or clinical diagnostic laboratories is 96-well with a typical reaction volume between 100 and 200 μL per well. Higher density microplates are typically used for screening applications, when throughput and assay cost per sample become critical parameters, with a typical assay volume between 5 and 50 μL per well. Common detection modes for microplate assays are absorbance, fluorescence intensity, luminescence, time-resolved fluorescence, and fluorescence polarization.
CellProfiler is free, open-source software designed to enable biologists without training in computer vision or programming to quantitatively measure phenotypes from thousands of images automatically. Advanced algorithms for image analysis are available as individual modules that can be placed in sequential order together to form a pipeline; the pipeline is then used to identify and measure biological objects and features in images, particularly those obtained through fluorescence microscopy.
In medicine, a biomarker is a measurable indicator of the severity or presence of some disease state. It may be defined as a "cellular, biochemical or molecular alteration in cells, tissues or fluids that can be measured and evaluated to indicate normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention." More generally a biomarker is anything that can be used as an indicator of a particular disease state or some other physiological state of an organism. According to the WHO, the indicator may be chemical, physical, or biological in nature - and the measurement may be functional, physiological, biochemical, cellular, or molecular.

In pharmacology, the term mechanism of action (MOA) refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect. A mechanism of action usually includes mention of the specific molecular targets to which the drug binds, such as an enzyme or receptor. Receptor sites have specific affinities for drugs based on the chemical structure of the drug, as well as the specific action that occurs there.

The single cell gel electrophoresis assay is an uncomplicated and sensitive technique for the detection of DNA damage at the level of the individual eukaryotic cell. It was first developed by Östling & Johansson in 1984 and later modified by Singh et al. in 1988. It has since increased in popularity as a standard technique for evaluation of DNA damage/repair, biomonitoring and genotoxicity testing. It involves the encapsulation of cells in a low-melting-point agarose suspension, lysis of the cells in neutral or alkaline (pH>13) conditions, and electrophoresis of the suspended lysed cells. The term "comet" refers to the pattern of DNA migration through the electrophoresis gel, which often resembles a comet.
High-content screening (HCS), also known as high-content analysis (HCA) or cellomics, is a method that is used in biological research and drug discovery to identify substances such as small molecules, peptides, or RNAi that alter the phenotype of a cell in a desired manner. Hence high content screening is a type of phenotypic screen conducted in cells involving the analysis of whole cells or components of cells with simultaneous readout of several parameters. HCS is related to high-throughput screening (HTS), in which thousands of compounds are tested in parallel for their activity in one or more biological assays, but involves assays of more complex cellular phenotypes as outputs. Phenotypic changes may include increases or decreases in the production of cellular products such as proteins and/or changes in the morphology of the cell. Hence HCA typically involves automated microscopy and image analysis. Unlike high-content analysis, high-content screening implies a level of throughput which is why the term "screening" differentiates HCS from HCA, which may be high in content but low in throughput.
Hit to lead (H2L) also known as lead generation is a stage in early drug discovery where small molecule hits from a high throughput screen (HTS) are evaluated and undergo limited optimization to identify promising lead compounds. These lead compounds undergo more extensive optimization in a subsequent step of drug discovery called lead optimization (LO). The drug discovery process generally follows the following path that includes a hit to lead stage:
High throughput biology is the use of automation equipment with classical cell biology techniques to address biological questions that are otherwise unattainable using conventional methods. It may incorporate techniques from optics, chemistry, biology or image analysis to permit rapid, highly parallel research into how cells function, interact with each other and how pathogens exploit them in disease.
Baculovirus gene transfer into Mammalian cells (BacMam) is the use of a baculovirus to deliver genes to mammalian cells. Baculoviruses are insect viruses that are typically not capable of infecting mammalian cells; however, they can be modified to express proteins in mammalian cells. Unmodified baculoviruses are able to enter mammalian cells; however, their genes are not expressed unless a recognizable mammalian promoter is incorporated upstream of a gene of interest. Both the unmodified baculovirus and its modified counterpart are unable to replicate in humans, making them non-infectious.
Phenotypic screening is a type of screening used in biological research and drug discovery to identify substances such as small molecules, peptides, or RNAi that alter the phenotype of a cell or an organism in a desired manner. Phenotypic screening must be followed up with identification and validation, often through the use of chemoproteomics, to identify the mechanisms through which a phenotypic hit works.
A 3D cell culture is an artificially created environment in which biological cells are permitted to grow or interact with their surroundings in all three dimensions. Unlike 2D environments, a 3D cell culture allows cells in vitro to grow in all directions, similar to how they would in vivo. These three-dimensional cultures are usually grown in bioreactors, small capsules in which the cells can grow into spheroids, or 3D cell colonies. Approximately 300 spheroids are usually cultured per bioreactor.
Chemoproteomics entails a broad array of techniques used to identify and interrogate protein-small molecule interactions. Chemoproteomics complements phenotypic drug discovery, a paradigm that aims to discover lead compounds on the basis of alleviating a disease phenotype, as opposed to target-based drug discovery, in which lead compounds are designed to interact with predetermined disease-driving biological targets. As phenotypic drug discovery assays do not provide confirmation of a compound's mechanism of action, chemoproteomics provides valuable follow-up strategies to narrow down potential targets and eventually validate a molecule's mechanism of action. Chemoproteomics also attempts to address the inherent challenge of drug promiscuity in small molecule drug discovery by analyzing protein-small molecule interactions on a proteome-wide scale. A major goal of chemoproteomics is to characterize the interactome of drug candidates to gain insight into mechanisms of off-target toxicity and polypharmacology.
Shantanu Chowdhury is an Indian structural biologist and a professor at Institute of Genomics and Integrative Biology of the Council of Scientific and Industrial Research. He is known for developing a mechanism for gene regulation mediated by DNA Secondary-Structure in diverse cellular contexts. An elected fellow of the National Academy of Sciences, India, he is a recipient of the National Bioscience Award for Career Development of the Department of Biotechnology in 2010. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 2012, for his contributions to biological sciences.
Cosimo Commisso is a Canadian cell biologist and cancer researcher who has made significant advances in the field of cellular trafficking and cancer metabolism. Among his most notable contributions are the discovery and study of how macropinocytosis supports tumor cell growth and survival by serving as an amino acid supply route in Ras-mutated cancers. He is currently an associate professor in the Tumor Initiation and Maintenance Program at the Sanford Burnham Prebys Medical Discovery Institute NCI-designated Cancer Center in La Jolla, California, USA.

Anne E. Carpenter is an American scientist in the field of image analysis for cell biology and artificial intelligence for drug discovery. She is the co-creator of CellProfiler, open-source software for high-throughput biological image analysis, and a co-inventor of the Cell Painting assay, a method for image-based profiling. She is an Institute Scientist and Senior Director of the Imaging Platform at the Broad Institute.









